
Puma Biotechnology and Specialised Therapeutics Asia Enter into Exclusive Licensing Agreement to Commercialize NERLYNX&r...

France based Step Pharma has announced the closing of a Series A financing totalling €14.5 million. Pontifax will ...
Singapore – RxSight (a US based medical devices company) announced that the U.S. Food and Drug Administration (FDA...

Japan based biopharma company PeptiDream Inc. has announced that it has entered into a multi-target discovery collaborat...
U.S. based Pharma Company Ionis Pharmaceuticals, Inc. has announced that it has licensed a second orally delivered Gener...

US based pharma company Cue Biopharma™, Inc. has announced a strategic strategic research collaboration and licens...

Pharma Major Lupin announced the launch of its Doxycycline Hyclate Tablets USP, 75 mg and 150 mg after an approval from ...

Pharma company Lupin reported its financial performance for the second quarter ending September 30th, 2017. The company...

Pharma Major Lupin has received final approval for its Testosterone Topical Solution, 30 mg per actuation from the Unite...

Pharma Major Lupin has announced that it has received final approval for its Carbidopa Tablets, 25 mg and Clonidine Hydr...

Biopharma company AstraZeneca has announced its US Food and Drug Administration (FDA) approved Bydureon BCise&...
Incidence of non-communicable diseases (NCDs) is on the rise, globally as well as in Asia Pacific region. Implications a...
Pharma Major Lupin has announced that it has received final approval for its Nadolol Tablets USP, 20 mg, 40 mg, and 80 m...

Singapore - Southeast Asia is home to 9 million heart failure patients. In Singapore alone, it accounts for around 6,000...

Started in 1996, October 12 is recognised as the World Arthritis Day (WAD). Established by Arthritis and Rheumatism Inte...

Michigan ranked third among 11 Midwest regions for the value of equity investments made in life sciences companies durin...

Eli Lilly and Company has decided to streamline operations to more efficiently focus resources on developing new medicin...

Pharma company Lupin Limited has announced the launch of its Olmesartan Medoxomil tablets, 5 mg, 20 mg and 40 mg having ...

Singapore – The U.S. Food and Drug Administration (FDA) approved Chemo Research's New Drug Application (NDA) for b...
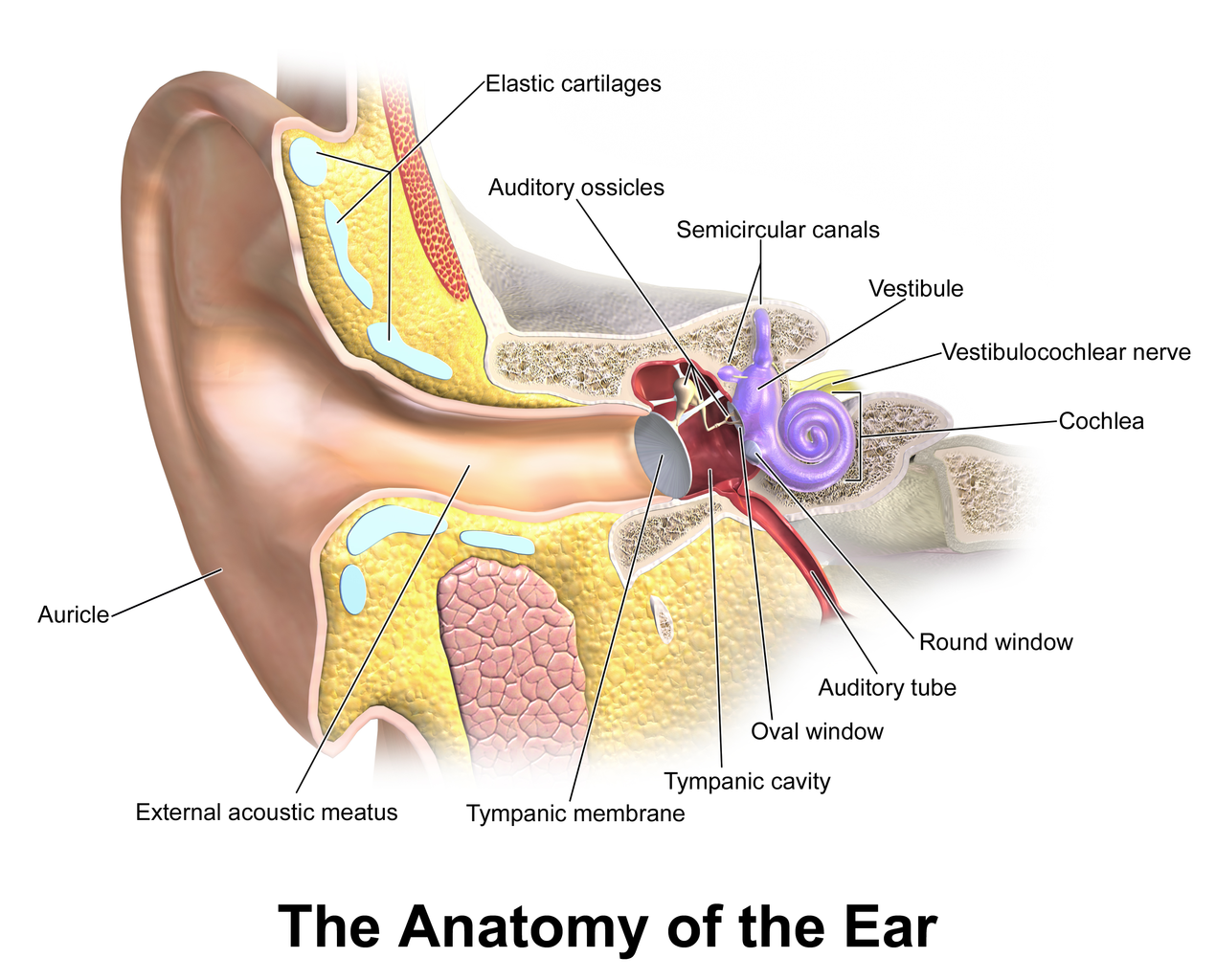
Singapore - With the pressing need for more efficient and agile pain management practices for military service per...
The U.S. Food and Drug Administration (FDA) has approved a new indication for Victoza (liraglutide) to reduce the r...

Lupin Limited has announced the launch of its Quetiapine Fumarate Extended-Release Tablets, 50 mg, 150 mg, 200 mg, 300 m...

Singapore – US FDA has granted two orphan drug designations (ODD) for T cell therapy products to Singapore-ba...

Singapore- As an additional measure in the fight against Zika virus, today the U.S. Food and Drug Administration announc...
Singapore – Bruker, a US based manufacturing company of scientific instruments announced that it has received US F...

LEO Pharma announced that the European Commission has granted marketing authorization for Kyntheum (brodalumab), a new b...

Merck announced in a press release that the U.S. Food and Drug Administration (FDA) has granted tentative appr...

Singapore - COPD hospitalizations are at an all-time high. The 30-day readmission rate for patients ranges from 20-39 pe...

Bringing in much needed relief for millions of gastroenteropancreatic neuroendocrine cancer patients, the Japanese regul...

Singpaore: In a bid to strengthen trade between two countries, the Taiwan Pharmaceutical Manufacturer’s Associatio...

Singapore: The European Commission has approved Xtandi (enzalutamide) capsules for the treatment of men with metastatic ...

Singapore: Sanofi's India unit and Carlyle group are in talks to buy the domestic drug formulation business of Indian dr...

Singapore: US Food and Drug Administration has raised alarm for Gilead Science's cancer medicine Zydelig (idelalisib) du...

Singapore: On July 9, 2012, the Generic Drug User Fee Amendments of 2012 (GDUFA) was enacted by the US. The Food and Dru...

Mumbai: Mumbai-based pharma major Lupin's subsidiary, Lupin Pharmaceuticals, has received approval for its Norgestimate ...

Singapore: The US Food and Drug Administration (FDA) has given its finally approval to India's Sun Pharmaceutical Indust...

Singapore: Miffed at Indian government's move to slash prices of essential medicines through the new drug pricing policy...

New Delhi: Ahead of the Drug Price Control Order (DPCO) to revise drug prices that came into effect on July 29, medicine...

Singapore: US Food and Drug Administration has approved the first generic versions of Cymbalta (duloxetine delayed-relea...

Singapore: Japan's drug major Takeda Pharmaceutical has said that they are set to finish the early stage human studies o...

Daiichi Sankyo Company Limited has announced that on Friday, June 19, its generics subsidiary, Daiichi Sankyo Espha, wil...
Singapore: In a recent move, the US Food and Drug Administration (FDA) has decided to extend the timeline for granting t...

Singapore: The US Food and Drug Administration has approved generic versions of the blood thinning drug Plavix (clopidog...
Singapore: The US FDA released a set of further current good manufacturing practices (cGMP) violations that have been no...

Singapore: Taiwan based TWi Pharmaceuticals, has launched generic version of Par Pharmaceutical's Megace ES (megestrol a...

Singapore: What started as an effort to lower trade barriers across 12 Pacific Rim nations, from the US and Canada to Vi...

Singapore: Initiative for Medicines, Access & Knowledge (I-MAK), a team of lawyers and scientists campaigning for gl...

Singapore: Teva Pharmaceuticals Industries, Israel, and Sun Pharmaceutical Industries, India, are going to pay a combine...

Japan's Daiichi Sankyo Company and Maruishi Pharmaceutical Co. Ltd have signed an agreement to collaborate on the commer...

Singapore: Israel-based Teva Pharmaceuticals has recieved favourable response in England and Wales regarding the validit...

Mumbai: The pharmaceutical industry will be widely impacted after India's National Pharmaceutical Pricing Authority (NPP...
Singapore: Antidepressant market in US, that peaked at USD12 billion in 2008, has lost USD2.6 billion due to expired pat...

Australia's Starpharma Holdings Ltd and Ansell Limited have announced that the Australian Olympic team will be provided ...

Singapore: US based Celgene has settled the litigation with India's Natco Pharma, Natco's US partner, Arrow Internationa...

Singapore: Three quarters of the population of China and India are diabetic. This translates to a patient pool of nearly...

Singapore: According to a research conducted by the National Bureau of Economic Research, some generic drugs sent from I...
The Japanese pharmaceutical market is the second largest pharma market in the world following on the close heels of the ...
The global biologics research and development arm of AstraZeneca, MedImmune LLC, has entered into an exclusive license a...

Singapore: US generics major Mylan has entered into a settlement agreement with OSI Pharmaceuticals, a subsidiary of Jap...

New Delhi: The Indian health ministry has banned the sale of three medicines after learning about the health risks assoc...

Singapore: Beximco Pharmaceuticals, a manufacturer of generic pharmaceutical products and active pharmaceutical ingredie...

Singapore: Glenmark Pharmaceuticals(Glenmark) has been granted final approval by the United States Food & Drug Admin...

Singapore: US-based Amneal pharmaceuticals announced the acquisition of Actavis pharmaceuticals' generic business in Aus...
Singapore: ANI Pharmaceuticals, a global specialty pharmaceutical company recently announced collaboration with IDT Aust...

Brickell Biotech Inc., a US-based clinical-stage pharmaceutical company has signed an exclusive license and development ...

Singapore: Indian drug major Lupin Pharmaceuticals is set to expand its global footprint by scouting for acquisitions ac...

Singapore: US treatment market for rheumatoid arthritis (RA) is set to increase in value from $6.4 billion in 2013 to $9...

Asia attracted a lot of interest from foreign multinational giants in the year 2013. This not only led to the increase i...

Singapore: Israeli generic drug giant Teva pharmaceuticals made an unsolicited offer to buy US-based Mylan NV for about ...

Singapore: Teva Pharmaceutical Industries' recent $40.1 billion offer to purchase Netherlands-based Mylan, which is cruc...
Singapore: World Health Organization has launched "Roadmap for childhood TB: towards zero deaths", in an effort to preve...

Singapore: Boehringer Ingelheim and Eli Lilly and Company have presented their results from two randomized phase III cli...

Singapore: Many leading Indian drug makers, that comprise a core part of the country's $1,000 bn worth pharmaceutical in...

Singapore: India's Drugs Controller General of India (DCGI) has said that under the country's Drugs and Cosmetics Act, i...

Singapore: Pfizer and China's Zhejiang Hisun Pharmaceuticals, a leading Chinese pharmaceutical company, announced the la...

Singapore: Teva Pharmaceutical has launched the generic equivalent to Abilify, aripiprazole tablets, an atypical antipsy...

Singapore: Indian pharmaceutical major, Sun Pharmaceutical Industries announced that the generic version of Prevacid by ...
Singapore: Israel-based Teva Pharmaceutical is going to pay $718 million to Israel Tax Authority to pay for the release ...

Singapore: Sandoz, a Novartis company, has recieved USFDA approval of Glatopa, a first generic version of Teva's Copaxon...

Singapore: The US FDA has provided its approval for a generic version of cancer drug Doxil that has been facing shortage...

Singapore: Hong Kong Trade Development Council (HKTDC) and Modernized Chinese Medicine International Association (MCMIA ...

Singapore: UK's drug regulatory body, Medicines and Healthcare products Regulatory Agency (MHRA) has recalled 16 drugs m...

Singapore: China's primary economic planning agency, the National Development and Reform Commission (NDRC) is in the pro...

Singapore: India-based Aurobindo Pharma in the US is recalling Gabapentin capsules, USP 300 mg 100-count bottles, at con...

Singapore: After being rapped by the US FDA and Indian drug regulator for lapses in good manufacturing practices (GMP), ...

Singapore: The Chinese police who have been investigating allegations of bribery against senior executives working at UK...

Singapore: Pneumonia remains the single biggest killer of children under the age of five globally, claiming the lives of...

Singapore: Sanofi has filed a lawsuit against the US FDA in hopes of preventing the regulatory agency from releasing pac...

Singapore: In the last one year, Actavis has been aggressive in expanding its presence in Asia and the company was on an...

Singapore: The US FDA has issued an alert on import of Ranbaxy Laboratories drug products that are manufactured at its ...

New Delhi: Spelling further problems for India's pharma giant Ranbaxy and its Japanese owners Daiichi Sankyo, Apollo Pha...

Singapore: A Frost & Sullivan (F&S) analysis finds that the Asian generic market is expected to grow at a compou...

Singapore: Taiwan-based TWi Pharmaceuticals has decided to sell its products in the US market through its own US subsidi...

Singapore: With increasing number of non-compliance issues being logged against Indian drugmakers by the US FDA, this ti...

Hyderabad: Dr. Reddy's Laboratories has launched Finasteride tablets (1 mg), a bioequivalent generic version of Propeci...

Singapore: US-based Amerigen Pharmaceuticals' Chinese subsidiary, Suzhou Amerigen Pharmaceuticals has received Chinese F...

Singapore: US based ANI Pharmaceuticals has acquired 22 previously marketed generic drug products from Teva Pharmaceutic...

Singapore: Expressing grave concern over the deteriorating intellectual property environment in India, the Pharmaceutica...

Singapore: The US Food and Drug Administration has approved the first generic version of the cancer drug Doxil (doxorubi...

Singapore: US generics firm, Prasco Laboratories, has entered into alliance with US subisdiary of Japanese pharmaceutica...



