
Singapore: India's leading pharma company, Jubilant Life Sciences received an approval from the US FDA for its anti-depr...

Singapore: US FDA has accepted the transfer of ownership of 23 generic drug products to Australia based IDT. Formalising...

Bangalore: The Supreme Court will take up the landmark case involving swiss drugmaker Novartis and India's patent office...

Singapore: GBI Research has highlighted that the introduction of generic statins has kick started a decline in the globa...

Singapore: For the first time, founder of leading Indian drugmaker Sun Pharmaceuticals, Mr Dilip Shanghvi has been named...

Singapore: US-based Mylan Pharmaceuticals has launched generic version of Pfizer's Vfend, Voriconazole for Oral Suspensi...
Bangalore: India's generics giant Cipla has won a landmark patent case against Swiss drug maker Roche in the Delhi High ...

Singapore: Posting a net profit of Rs 1391 crores in Q1, Sun Pharma exhibited a flat growth in line with the market expe...

Singapore: The US Supreme Court declined a request from Teva Pharmaceutical for a stay of an appeals court ruling that w...
Singapore: Daiichi Sankyo's generic subsidiary, Daiichi Sankyo Espha has submitted a supplemental New Drug Application (...

Mumbai: India-based Aurobindo Pharma intends to acquire Actavis' generics commercial operations in seven markets in West...

Singapore: Dublin headquartered generic drugmaker Actavis has now been caught in a legal tangle after Indian drug major ...

New Delhi: In yet another blow to multinational companies operating in India, the Intellectual Property Appellate Board ...

Singapore: Taiwan based TWi Pharmaceuticals has appointed Ms Tina Guilder as President and CEO and Ms Eva Ho as CFO to s...

Singapore: Gland Pharma, a leading Indian pure-play generic injectable pharmaceutical products company, has signed an ag...

It has added one more feather to its cap by being the nesting ground to churn out new start-ups. It is an informal incub...

Singapore: The Federation of Asian Pharmaceuticals Association (FAPA) said that the upcoming patent cliff will be advant...

Singapore: With biosimilars promising an exponential growth, South Korea's Celltrion has filed an FDA application for Re...

Singapore: Britain's drug regulator, the UK Medicines and Healthcare Products Regulatory Agency has revoked the quality ...

Mumbai: Indian generic pharmaceuticals company Cipla appointed Mr Subhanu Saxena as its chief executive officer. Mr Saxe...

Singapore: Indian pharma major, Cipla recently announced that the company's wholly owned subsidiary Meditab holdings ent...

Singapore: Indian drug major Lupin has inked a marketing pact with South Korea's LG Life Sciences to launch its anti-di...

Singapore: British drug maker GlaxoSmithKline is in advanced talks to recruit Sir Philip Hampton, chairman of Royal Bank...

Singapore: India based generic drug maker Cipla has recieved FDA tentative approval for a generic formulation of emtrici...

Singapore: The US FDA has granted approval for the launch of Dr. Reddy's Laboratories's cancer drug Azacitidine for inje...

Singapore: India-based Aurobindo Pharma eyes on globalization and innovation to achieve a turnover of $2 billion in the...

Singapore: The birth of one of the largest generic pharmaceutical companies in South Korea just came about when Alvogen ...

Singapore: India's Aurobindo Pharma received final approval from the US FDA to manufacture and market Escitalopram Oxala...

Singapore: Canada has quarantined the health products made with active pharmaceutical ingredients (APIs) from India's Sr...

Singapore: Shanghai Fosun Pharmaceutical has been listed in CCTV Financial 50 Index Sample Stock, an index created by Sh...

Singapore: The US FDA has approved world's first generic version of Actos (pioglitazone hydrochloride) tablets. US-based...

The year 2011 was a year of capturing potential markets and achieving new milestones. Ranbaxy Laboratories became the fi...

Singapore: Interpol's sixth and the biggest annual worldwide crackdown, Pangea VI has ended in seizing of $41 million wo...

Singapore: Gilead have allowed generic manufacturers in India and China to produce generic versions of its HBV and HIV d...

Singapore: The Indian pharmaceutical industry is estimated to continue to experience strong growth in the near-term alth...

Singapore: India's pharmaceutical firm Dr. Reddy's has appointed Dr. Sripada Chandrasekhar as the president and global h...

Singapore: China-based Zhejiang Huahai Pharma received approval from the China FDA to produce efavirenz. Huahai is the f...

Singapore: An Indian news report that appeared in Business Line quoted an unidentified DIPP official who claimed that to...

Singapore: Leading pharma giant Merck has reinforced its commitments towards China, in a bid to strengthen its position ...

Mumbai: NATCO Pharma has filed a lawsuit against Mr Shamnaad Basheer, professor, West Bengal National University of Juri...

Singapore: Global generics firm, Actavis, has opened its regional office in Singapore that will serve as the headquarter...

Bangalore: As a first step to prevent misuse of drugs, which have been banned abroad from being sold freely in the count...
Multinational pharmaceutical company Mylan sued the US Food and Drug Administration (FDA) for approval to sell a generic...

Singapore: Ranbaxy Laboratories, which is already reeling under several regulatory rebukes owing to quality apprehension...

Singapore: The epilepsy therapeutics market value in the Asia-Pacific (APAC) region, Australia, China, India and Japan, ...

Singapore: Pfizer has settled its litigation against Mylan and Mylan Pharmaceuticals relating to Pfizer's patents coveri...

Singapore: Mundipharma has signed an exclusive agreement with the Swiss subsidiary of Horizon Pharma for the commerciali...

Singapore: Aurobindo Pharma has received US FDA approval for Repaglinide tablets, the generic equivalent of Novo Nordisk...
The year 2012 has brought a world of opportunities for Indian biotech companies, with big-wig biotech firms in the West ...
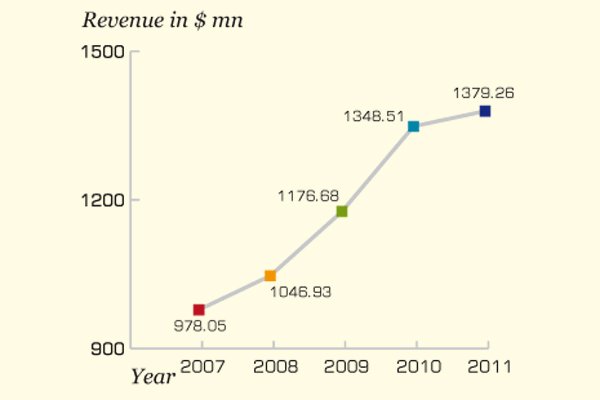
The king of generics, Cipla, has been aggressive in expanding product portfolio and reaching out to international market...

Singapore: HIV drug developer, Gilead Sciences has partnered with Medicines Patent Pool (MPP) to expand access to Gilead...

Bangalore: The Intellectual Property Appellate Board in Chennai, India, has rejected pharma major Bayer's petition seeki...

Singapore: Irish firm, Perrigo, has declined the acquisition offer from Mylan revalued at USD60 per share in cash and 2....
Singapore: The European Commission (EC) is going to fine seven generic drug firms, including India-based Ranbaxy and Den...

Singapore: Regulatory hurdles continue to haunt Impax Laboratories and its experimental drug, Rytary, that treats idiopa...

Singapore: The US FDA provided its final approval to Indian drug maker Lupin to manufacture and market a generic version...
Singapore: India's Aurobindo Pharma has submitted an Abbreviated New Drug Application (ANDA) for dolutegravir 50mg, for ...

Singapore: Montreal-based generic drug developer and marketer Pharmascience is venturing into Korea by forming a joint v...

Singapore: Orexigen Therapeutics and Takeda Pharmaceuticals have filed a lawsuit in US against Actavis for filing an abb...

Singapore: The US Food and Drug Administration (FDA) has slapped a warning letter to China based Tianjin Zhongan Pharma...

Singapore: Beximco pharmaceuticals, a Bangladeshi pharma giant announced that it had received Good Manufacturing Practic...

Singapore: With the strategy to strengthen its pain management portfolio, global biopharmaceutical company, Teva Pharmac...

Singapore: Australian drug maker Mayne Pharma announced Mr Blake Cullen as the new vice president of business developmen...

Singapore: British drugmaker GlaxoSmithKline has approached the Foreign Investment Promotion Board (FIPB) to hike stake ...
India's pharma industry, mainly comprising generics has got a breather. The government in India has decided to cap brown...

Singapore: Addressing the global media for the first time after allegations were leveled out against Britain's largest d...

Mumbai: Lupin Phamaceuticals has received final approval for its Ranolazine extended-release tablets, 500 mg and 1000 mg...

Singapore: Eli Lilly has filed a $500 million international lawsuit against the Canadian government alleging that the co...

Singapore: Alchemia's commercialization partner Dr Reddy's Laboratories has launched Alchemia's FDA approved generic an...

Singapore: Pharma major Abbott Laboratories has unveiled its strategy for the Indian market, claiming that it will push ...

Singapore: Global pharma firm, Pfizer, plans to acquire InnoPharma at approx $360 million to strengthen its generics, in...
Bangalore: Mylan Pharmaceuticals, a subsidiary of Mylan, has commenced commercial operations in India, starting with the...

Singapore: Asian pharmaceutical firm, Mundipharma, has promoted the general manager of Mundipharma Korea, Mr John Lee, t...

Singapore: Dr Reddy's Laboratories has launched sedative drug, Eszopiclone tablets (C-IV) 1 mg, 2 mg and 3 mg, a therape...

Japan, which has the lowest infant mortality rate and highest adult life expectancy in the world, is fast turning into a...
Singapore: The US Food and Drug Administration (FDA) has approved or tentatively approved a total of 152 antiretroviral ...

Mumbai: Pharmaceutical and biotechnology major Wockhardt has received final approval from the US Food & Drug Adminis...

Singapore: In what has come as another shocker for the Indian Pharmaceutical industry, the US FDA slammed RPG Life Scien...

India-headquartered Dr Reddy's Laboratories is making a strong name in biosimilars business in India and in the world....

Singapore: India based generics firm Sun Pharmaceutical Industries has announced to acquire Ranbaxy Laboratories through...

Bangalore: Dr. Reddy's Laboratories has launched Ibandronate sodium tablets (150 mg), a bioequivalent generic version of...
Singapore: Foraying into South American pharmaceutical market, India based Lupin Pharmaceutical has acquired Mexico firm...

Singapore: As Asia emerges as a major engine of global economic growth, the pharmaceutical and biotechnology industries ...

Singapore: Pharmaniaga Berhad, the largest integrated local healthcare company in Malaysia, reported a jump of 51 percen...

Singapore: Glenmark Pharmaceuticals has consolidated $254.45 million for the third quarter ending December 31, 2012, mar...
Healthcare systems, globally, are grappling with different facets of the same problem: How to provide affordable healthc...

Singapore: Raising concerns related to the fate of people who cannot afford expensive branded medicines, India's parliam...

Thecase of Indian pharma company's request for a compulsory license to make a generic version of Bayer's patented drug t...

Singapore: Even six days after Chinese police detailed some senior executives working at the Chinese office of the bigge...

Singapore: In what looked like a patent suit that could stir further controversy about patenting in the life sciences in...

A rapidly aging population has burdened the healthcare system both in terms of funding and facilities and is of great co...

Singapore: A day after Chinese police made the names of those involved in one of the country's biggest scandal involving...

New Delhi: In what is being criticized as yet another miserable experience created by India's intellectual property and ...

Singapore: The Indian Drug Controller General of India (DCGI) has written a letter to the drug maker Wockhardt to explai...

Singapore: Dr. Reddy's Laboratories has launched drug for psychiatric conditions, Divalproex Sodium extended release tab...

Singapore: FDA has granted tentative approval to India-based Strides Arcolab's HIV drug emtricitabine and tenofovir diso...

Singapore: Days after a probe was initiated by the Chinese police into alleged 'economic crimes' committed by senior off...

Bangalore: Ranbaxy Pharmaceuticals, a wholly owned subsidiary of Ranbaxy Laboratories, has launched authorized generic ...

Singapore: After acquiring the formulations business of India's Piramal Healthcare in 2010, US drug major Abbott Laborat...
Singapore: China-based Tianyin Pharmaceutical (TPI) received GMP certification for its pre-extraction plant and formula...



