
Japanese pharmaceutical firm Eisai Co.,Ltd. has announced that it has entered into a collaboration research agreement wi...
CEL-SCI Corporation (NYSE American: CVM) announced that one of its key collaborators from Rush University Medi...
Gilead Sciences, Inc., on 24 June 2019, announced that the Singapore Health Sciences Authority (HAS) has approved Vosevi...

Gilead Sciences and Nurix Therapeutics, Inc., have announced a global strategic collaboration to discover, dev...

Zydus’ Nesher Pharmaceuticals, a subsidiary of Zydus Pharmaceuticals USA has received the final approval from the ...
Acucela Inc. (“Acucela”), a clinical-stage ophthalmology company and wholly-owned subsidiary of Kubota Pharm...

Sosei Group Corporation and its strategic alliance partner Pfizer announce that a new clinical candidate from their mult...

CStone Pharmaceuticals, on 9 June 2019, announced its global clinical collaboration with China focus with Baye...
Rykindo® (LY03004), Luye Pharma's innovative, independently developed Extended-Release Microspheres f...

Sihuan Pharmaceutical Holdings Group Ltd., the largest cardio-cerebral vascular (CCV) drug manufacturer in China's&...

BGN Technologies, the technology transfer company of Ben-Gurion University (BGU), introduces a novel drug comb...

Gilead Sciences, Inc. has recently announced that with effect from 1 May 2019, Vemlidy (tenofovir alafenamide, TAF)...

ADC Therapeutics, an oncology drug discovery and development company that specializes in the development of proprietary ...
Roche has declared that Emicizumab (Hemlibra®) has been approved in India for Hemophilia A with factor VIII in...

Eisai Co., Ltd. is a leading global research and development-based pharmaceutical company headquartered in Japan. The co...
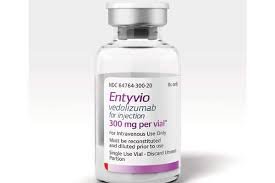
Takeda Pharmaceutical Company Limited announced that the European Medicines Agency (EMA) has accepted a Marketing Author...

Standigm, a company using artificial intelligence (AI) technology for drug discovery and development, announced it has r...

StrideBio, Inc, a leading developer of novel adeno-associated viral (AAV) based gene therapies, recently announced the s...



