Emmaus Life Sciences Announces Support of Sickle Cell Disease Legislation
02 August 2018 | News
Bill Aims to Improve Understanding of Health Care System Utilization for Patients with SCD.
Emmaus Life Sciences, Inc. has announced its support of the Sickle Cell Disease Surveillance, Prevention, and Treatment Act of 2018 (S. 2465). The legislation seeks to improve the understanding of health care system utilization patterns of people with sickle cell disease (SCD) and establish cost-effective practices to improve and extend the lives of these patients.
In July 25, the Senate Health, Education, Labor, and Pensions (HELP) Committee unanimously approved S. 2465, the Sickle Cell Disease Research, Surveillance, Prevention and Treatment Act of 2018. Next, it will be reviewed by the full Senate.
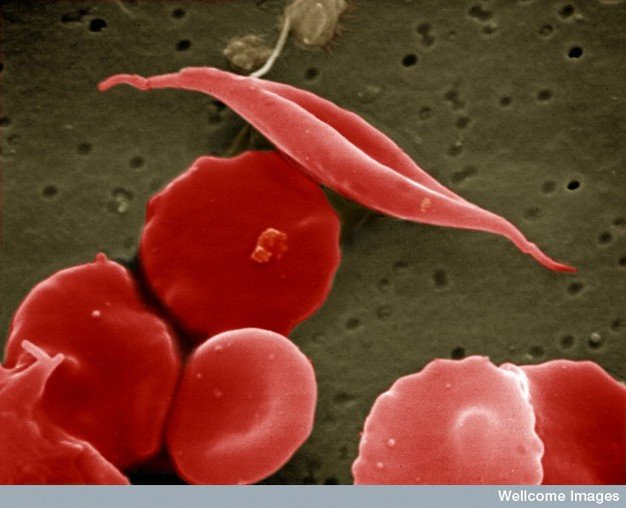
Sickle cell disease is an inherited blood disorder characterized by the production of an altered form of hemoglobin which polymerizes and becomes fibrous, causing red blood cells to become rigid and change form so they appear sickle shaped instead of soft and rounded. Patients with sickle cell disease suffer from debilitating episodes of sickle cell crises, which occur when the rigid, adhesive and inflexible red blood cells occlude blood vessels. Sickle cell crises cause excruciating pain as a result of insufficient oxygen being delivered to tissue, referred to as tissue ischemia, and inflammation. These events may lead to a variety of other adverse outcomes such as acute chest syndrome that requires hospitalization. Sickle cell disease is an orphan disease, affecting approximately 100,000 patients in the U.S. and millions worldwide, with significant unmet medical needs.
“As a physician who has treated SCD patients for more than twenty years, I know first-hand the impact of this disease,” said Yutaka Niihara, MD, MPH, CEO and founder of Emmaus Life Sciences. “This condition exacts a terrible toll on patients, their families and the community in terms of pain, missing school or work and hospitalizations. We are pleased to see this legislation and look forward to it being passed.”
Juanita Gougis added, “As a patient with sickle cell disease, I am adding my voice in support of this bill and its recognition of the importance of a better understanding of the prevalence of SCD and its associated health implications.”
“On July 25, the Senate Health, Education, Labor, and Pensions (HELP) Committee unanimously approved S. 2465, the Sickle Cell Disease Research, Surveillance, Prevention and Treatment Act of 2018,” said Beverley Francis-Gibson, president/CEO for the Sickle Cell Disease Association of America, Inc. “This is wonderful news, and we are grateful for the efforts of Senators Tim Scott (R-SC) and Cory Booker (D-NJ), who introduced the bill. However, we must continue our collaborative advocacy efforts to address the complexities of sickle cell disease and to ensure that the bill becomes law.”
Endari (L-glutamine oral powder), Emmaus’ first commercially available product, was approved in July 2017 by the FDA and was the first treatment in nearly 20 years for sickle cell disease. Endari has received Orphan Drug designation in the U.S., and Orphan Medicinal Product designation in the EU.
On July 18, 2018, Emmaus announced that the New England Journal of Medicine published results of the company’s 48-week, phase 3 clinical trial of Endari, which supported the FDA approval in July 2017.












