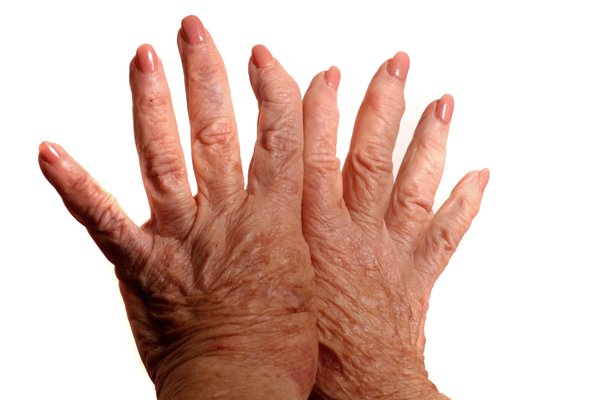
Iguratimod demonstrated superiority over placebo and non-inferiority compared to an existing DMARD in a study
Singapore: Eisai and Toyama Chemical have received approval from Japan's Ministry of Health, Labour and Welfare to market iguratimod for the treatment of rheumatoid arthritis.
Iguratimod, originally discovered by Toyama Chemical, is a novel disease modifying anti-rheumatic drug (DMARD) jointly developed in Japan by Eisai and Toyama Chemical based on a co-development and license agreement previously concluded between the two companies.
In a clinical study of iguratimod administered as a monotherapy in patients with rheumatoid arthritis, the agent demonstrated superiority over placebo and non-inferiority compared to an existing DMARD (salazosulfapyridine). In addition, in a trial of iguratimod in combination with methotrexate (MTX), the standard of care, conducted in rheumatoid arthritis patients who did not achieve satisfactory benefit with MTX alone, patients who were administered a combination of the two agents demonstrated favorable tolerability as well as significant improvements compared to those treated with placebo (MTX-only arm) in the study's primary endpoint of ACR20 response rate at Week 24. Out of all the orally-administered anti-rheumatic drugs currently approved in Japan, iguratimod is the first agent evaluated in domestic clinical trials to demonstrate efficacy as an add-on therapy to MTX in patients who did not achieve satisfactory benefit with MTX alone.
Once listed on Japan's National Health Insurance (NHI) drug price list, iguratimod will be sold by Eisai and Taisho Toyama Pharmaceutical under the brand names Careram Tablets 25 mg and Kolbet Tablets 25 mg, respectively, with both companies working to market the product and provide information on its proper use. Following launch, the two companies will also conduct a special use results survey (all-case surveillance) in all patients who are administered the drug until a pre-determined number of patients has been reached.
By providing iguratimod as a new option for the pharmacological treatment of rheumatoid arthritis, Eisai and Toyama Chemical believe that they will be able to make further contributions to address the diversified needs and improve the quality of life of rheumatoid arthritis patients.




