Researchers from the Kirby Institute at UNSW Sydney, Australia together with colleagues in the United States and Europe are leading a multinational COVID-19 clinical trial funded by the United States National Institutes of Health (NIH), announced this month by Dr Anthony Fauci, Director of the U.S. National Institute of Allergy and Infectious Diseases.
The trial, called INSIGHT 013 or ITAC (Inpatient Treatment with Anti-Coronavirus Immunoglobulin), will test ‘hyperimmune immunoglobulin’ treatments in individuals hospitalised with COVID-19.
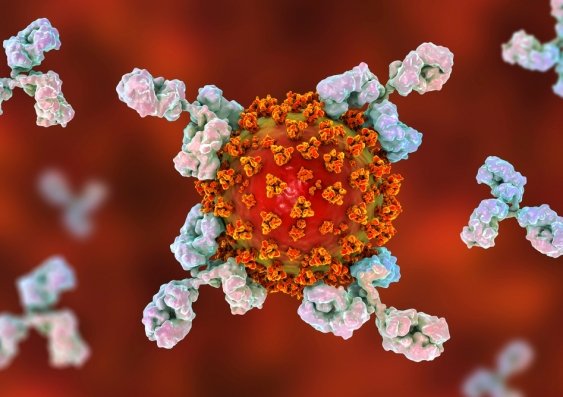
“Hyperimmune immunoglobulins are antibodies purified from plasma taken from people who have recovered from COVID-19. For ITAC, antibodies from up to 50 recovered individuals are pooled in the final product, which is designed to enhance the immune response to the virus and block its entry into cells,” says Associate Professor Mark Polizzotto, who is protocol chair of the ITAC trial.
The ITAC trial will be conducted at more than 60 sites across 12 countries. It is a randomised, double blinded, controlled platform trial of 500 patients, which will compare the effectiveness of hyperimmune immunoglobulin to placebo. All patients will receive standard-of-care therapies including remdesivir, an anti-viral drug shown to shorten recovery times in COVID-19 in another NIH-sponsored trial.
Four companies are collaborating to provide anti-coronavirus hyperimmune immunoglobulin for the trial: Emergent BioSolutions; Grifols S.A.; CSL Behring; and Takeda Pharmaceuticals.
“This trial is a world-first in a number of ways,” said A/Prof. Polizzotto. “It’s the first time we’re testing the efficacy of this promising treatment in patients with COVID-19. It is also remarkable that four major pharmaceutical companies have come together to participate together in a single clinical trial. This exceptional, global scientific collaboration involving the public and private sector is a coordinated response to the seriousness of COVID-19.”
ITAC is being conducted by the INSIGHT network, a partnership of five institutions across the United States, Europe and Australia coordinated by the University of Minnesota. The Kirby Institute will coordinate trial implementation at sites in Asia, parts of Africa and the Middle East, and Latin America. Recruitment has begun, with results expected later in 2020.
Australian ‘INSIGHT’ from HIV is facilitating a rapid COVID-19 response
ITAC is one of a number of COVID-19 treatment and observational trials launched by the INSIGHT (the International Network of Strategic Initiatives in Global HIV Trials) network since the COVID-19 pandemic began. The unprecedented pace of trial development, shortening a process that usually take years to a matter of months, reflects years of preparation by network leaders. INSIGHT conducted pivotal trials in HIV and other viral infections including influenza since 20 years.