Singapore - Philip Morris International (PMI), has conducted an in vitro study using epithelial cells taken from the mouth and small airways of a human donor, in early stage, pre-clinical assessment of IQOS MESH, a unique electronic cigarette (e-cigarette). Results of the study have been presented at the 20th International Congress on In Vitro Toxicology (ESTIV) in Berlin, Germany.
Cigarette smoking causes a number of serious diseases and increases the risk of early death. The most effective way to mitigate these risks is to never start smoking or to quit, but for existing adult smokers who would otherwise continue to smoke cigarettes, electronic alternatives may reduce their risk of harm. However, the biological effects of e-cigarette use can only be fully understood through rigorous, robust, and transparent scientific assessment. The in vitro study presented at ESTIV represents part of the early stages of this assessment for IQOS MESH.
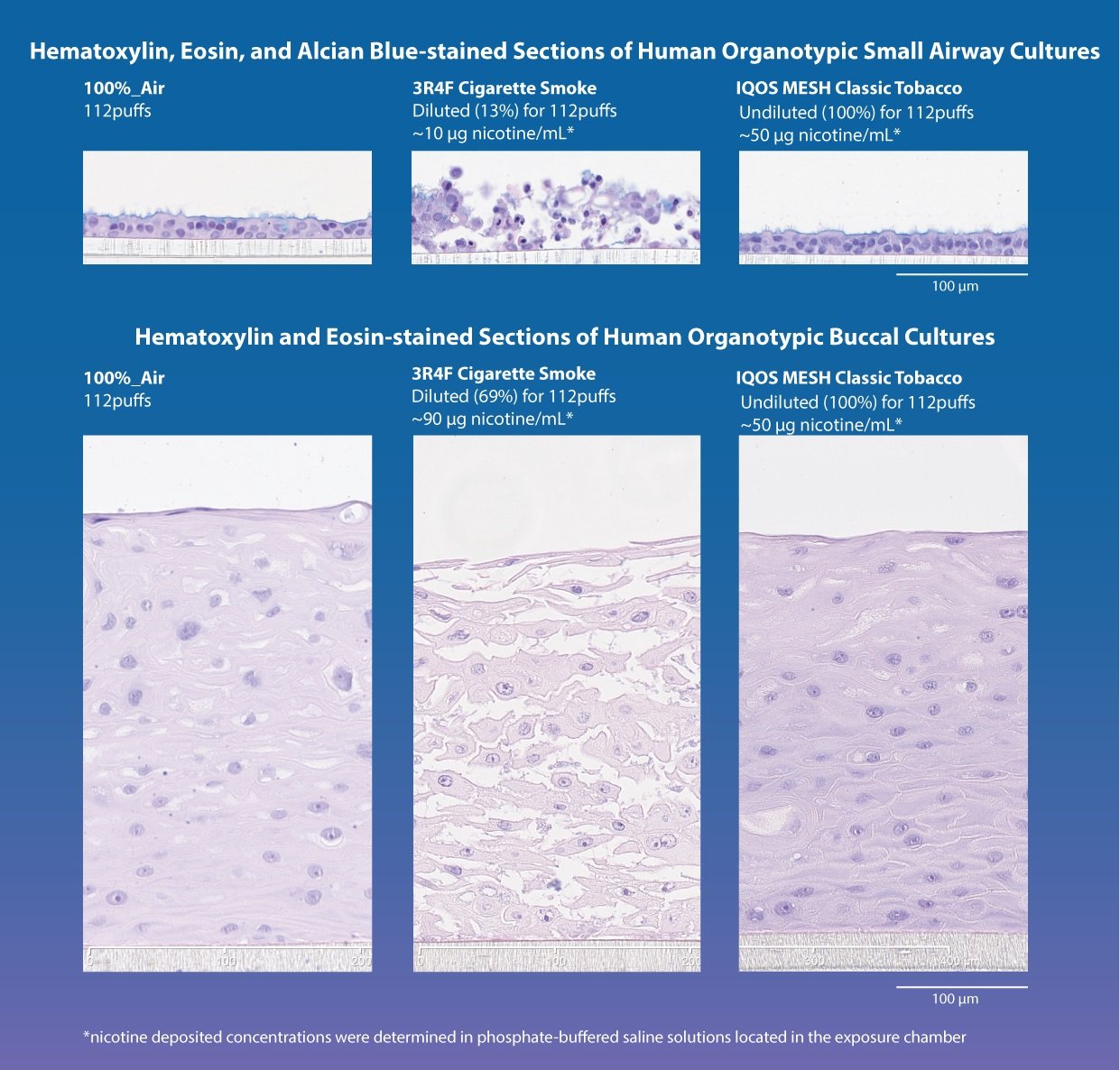
Donated cells were reconstituted in three-dimensional (3D) cultures, allowing them to grow and develop in a manner that closely resembles human physiology. 3D in vitro studies of this sort can facilitate the pre-clinical assessment of potential toxins without the need for animal models, and therefore support the internationally recognized “3Rs” of animal research: “replacement, reduction, and refinement.” Using an industry standard exposure system, cells were exposed to either IQOS MESH aerosols, cigarette smoke, or fresh air. Three different types of IQOS MESH aerosols were used: 1) a “classic” formulation containing both flavorings and nicotine, 2) aerosol containing nicotine, but no flavorings, 3) aerosol containing no nicotine and no flavorings. Exposures of IQOS MESH aerosols and cigarette smoke were matched for number of puffs, duration, and, where relevant, comparable concentrations of nicotine. Cultures were collected 48 hours after exposure for analysis and the study was conducted over three experimental repetitions. While most in vitro exposure studies look at molecular endpoints, they do not examine the structural and functional effects of cell exposure (morphology), as was the case here.
Analysis of cell morphology demonstrated that all IQOS MESH aerosols, regardless of the presence of nicotine and flavorings, had minimal or no impact on epithelial cells of the mouth and small airways. Overall, morphology of the cultures exposed to IQOS MESH aerosols was comparable with that of the cultures exposed to fresh air alone. In comparison, epithelial cells taken from the mouth that were exposed to cigarette smoke showed marked tissue disintegration, while epithelial cells taken from the small airways that were exposed to cigarette smoke exhibited degeneration and loss of cilia (slender projects from the cell body which play a vital role in respiratory health).
IQOS MESH is a unique, two-part e-cigarette combining a fixed device and a consumable cap. It is designed to address some of the challenges presented by e-cigarettes currently on the market and to ensure the consistency and quality of the generated aerosol. Classic e-cigarettes use a “coil-wick” system to heat e-liquids, while in IQOS MESH the wick is eliminated and replaced by a metal mesh. The mesh has a larger surface area than coil-wicks, and thus heats the solution in a more consistent way. With currently available e-cigarettes the temperature of the heater can also vary significantly, depending on how strongly the e-cigarette is puffed, while with IQOS MESH the temperature of the heater is maintained between 200-220°C. In addition, when the e-liquid in IQOS MESH reaches a low level, the heating is automatically stopped, preventing “dry puffs” which create higher levels of harmful chemicals.
The scientific assessment of the biological impact of IQOS MESH is ongoing and it is expected that further studies will present in the future. It one of a portfolio of Reduced-Risk Products (RRPs)* being developed by PMI. Through technological innovation and rigorous scientific assessment, PMI is leading a full-scale effort to ensure RRPs ultimately replace cigarettes.




