Singapore: Australian drug company Agenix received a key patent covering the manufacturing process of its ThromboView im...
Singapore: The fifth QVA149 (indacaterol maleate / glycopyrronium bromide) phase III study of Novartis, SPARK, met its p...

Singapore: Biotech company ArQule and Japan-based Daiichi Sankyo have achieved positive results from a randomized, place...

Singapore: Tokyo-headquartered Eisai has signed a global agreement with Brazil's Fundação Oswaldo Cruz for collabo...

Singapore: Qualyst Transporter Solutions entered an alliance with GenoMembrane, Kanagawa, Japan, to make available its ...

Singapore: Pieris received the first financial milestone in its discovery and development collaboration with Daiichi Sa...

Singapore: Zoll Medical, a manufacturer of medical devices and related software solutions, has received Shonin approval ...

Singapore: SBI Pharmaceuticals, headquartered in Japan, has concluded a basic agreement to begin clinical research on di...

Singapore: Thailand's Nanotec signed a collaborative Letter of Intent (LoI) with the Nanosystem Research Institute (NRI)...

Singapore: Takeda Oncology and the Worldwide Innovative Network (WIN) in personalized cancer medicine consortium have fo...

Singapore: An investigator-sponsored clinical study in Japan using Cytori's Celution System for treatment of cryptoglan...

Singapore: Oxford Immunotec, a medical diagnostic company developing tests in the fields of infectious and immunologica...

Singapore: Medtronic launched the Resolute Integrity Coronary Stent System in Japan, which is the world's second largest...

A rapidly aging population has burdened the healthcare system both in terms of funding and facilities and is of great co...

Singapore: Clearbridge BioMedics has won the 2012 Asian Entrepreneurship Awards, which was held from May 9-11, 2012, in ...

Singapore: India-based Elder Pharmaceuticals is said to be up for sale, according to a report published in the Economic ...

Singapore: Astellas Pharma is constructing a new building at the Takahagi Technology Center in Japan. The building, whic...
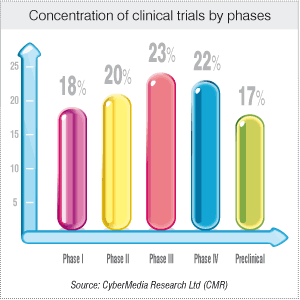
The fifth edition of the annual BioSpectrum Asia Pacific clinical research organization (CRO) Survey, which is conduct...



