Starlims’ 36 years of evolutional technology supports laboratories in their digital transformation
27 August 2021 | Analysis
Digital transformation is a term that is hard to define concisely, but for organizations in multiple industries, it can be defined as the adoption of a data management infrastructure that allows their laboratories to work faster and more productively, with greater transparency.

More than just working towards a paperless lab, digital transformation may allow companies to derive more intelligence from their data, ultimately improve product and process safety, and enable faster regulatory audit or approval timelines. At the level of data that labs generate and interact with, smart digital solutions should help to safeguard data integrity, and make data more accessible, contextual, and usable over the long term.
STARLIMS, one of the global Laboratory Information Management Systems (LIMS) leaders with over 36 years in the market, is a mission critical application that supports this digital transformation from the ground up, while addressing the issues of “technical debt.” As laboratory instruments, platforms and software are updated and replaced, outdated systems are retired from use, but many will still need to be maintained at significant cost in order to access data held in proprietary formats. It's a fact of laboratory life that has existed within the LIMS industry for over 36 years.
The prospect of technical debt should represent a caveat for laboratories that are chasing the latest, ‘revolutionary’ vendor-locked technologies, which may promise to support digital transformation in the short term but may result in technical debt five or ten years down the line. In contrast, STARLIMS has taken an evolutionary approach to technological development, so that it can grow and adapt in parallel with the data requirements, formats and requirements of laboratories taking on new analytical technologies and workflows. It’s about empowering manufacturing, R&D and testing laboratories with the ability to negotiate the rapidly evolving technological and data landscape seamlessly, so that they can protect their investment and derive maximum insight from virtually every piece of data, enterprise wide. STARLIMS aims to constantly innovate. In the world of technology especially data management, staying ahead of the innovation curve and anticipating future customer needs is key.

STARLIMS Digital Assistance Technology
“STARLIMS has taken an evolutionary, rather than a revolutionary path to digital transformation”. Revolutionary approaches can require building entirely new platforms. In contrast, an evolutionary stance to product development understands and protects customers’ investments, guards their data, and allows them to leverage new technologies stepwise and derive a strong return on their investments. Importantly, customers shouldn’t have to replace systems that become outdated or tie themselves to the costs associated with maintaining legacy systems.
This evolutionary approach to product development has been embedded in STARLIMS since its establishment more than 36 years ago. STARLIMS helped to invent the industry. The foundation of the company was rooted in the critical need for laboratory quality assurance and quality control testing, and the data management tools. One of STARLIMS first largest global client was petrochemicals company Chevron, which carried out testing in its refineries.
The STARLIMS concept was from the start focused on developing an agile informatics platform that would automate the QC lab testing workflow, to ensure the quality and efficacy of manufactured products. Technologically, it has evolved its offerings from the adoption of early PC-based systems and early web-based platforms, to current-day hosted, cloud solutions, mobile computing and the development of applications based on HTML5.
Product development of STARLIMS today marries extensive capabilities in data management and integration, artificial intelligence-driven analytics, virtualization and visualization, and the massive capacity of cloud computing through Amazon Web Services. And while the platform is used across multiple industries, today, it sees the largest growth opportunities for the STARLIMS suite of solutions across the life sciences, from pharma manufacturing QC to outsourced testing, and R&D.
For companies in this sector, challenges include the need, on the one hand, to digitize and make legacy data accessible and searchable so that drug development workflows that were undertaken generated decades ago are still relevant going forwards, and on the other hand to incorporate the latest high-throughput technologies, such as next-generation sequencing, and other high–throughput and high content workflows, into the drug development and testing processes. STARLIMS has been developed to adapt to the particular challenges of the life sciences industry, which has to manage data from laboratory testing across the four pillars of a product life cycle; research and preclinical, clinical, manufacturing and post-market surveillance, in the global context of that product’s development.
STARLIMS is designed to interface and integrate with an organization’s existing instrumentation and software solutions and platforms, across an enterprise, both locally and globally. This facilitates the secure, safe and fully traceable exchange of disparate data types across laboratories and continents, throughout the product lifecycle. And as organizations adopt new technologies and processes, STARLIMS can directly acquire a diverse range of instrument data. Through the STARLIMS SDMS [scientific data management system] customers can aggregate data and produce an audit trail from the original sample through all the instrumentation, and then be able to review and make actionable decisions based on that data for quality purposes, audit purposes and longitudinal history. STARLIMS allows customers to harmonize that flow of data through multiple plants from an operational, procedural, and quality management perspective, to comply with GLP and CFR 21 part 11 requirements.
The development of STARLIMS has been focused on easing the regulatory burden by automating the collation of raw and standardized data, together with generating requisite reports and generating searchable audit trails. STARLIMS has certification for how it manufactures its product and the level of quality around data security. One of the greatest costs to the customer in implementing a LIMS is user validation. STARLIMS’ multi-layered platform allows upgrade of the infrastructure without changing the user experience, while limiting the amount of user training that needs to take place. That return on cost of ownership increases, and the ability to pass on to customers the cost savings associated with purchasing computing power through Amazon Web Services further reduces their own internal IT burden.
Importantly, and particularly in the QC arena, STARLIMS’ sophisticated analytics suites and visualization tools are transforming laboratories into predictive, rather than reactive environments. Artificial Intelligence guided algorithms can model future processes, predict bottlenecks and highlight trends that may lead to batch failures, based on real-time data. STARLIMS’ advanced analytics can then uncover where potential and actual process, instrument, or workflow faults lie, identify areas for improvement or resolution.
STARLIMS’ advanced analytics and predictive modelling give QC and manufacturing labs the ability to analyze the details of their key processes and workflows, quality and productivity issues, and ancillary factors, from material supply chain, to current and predicted capacity, and allocation of personnel time. The bottom line is ensuring quality and expedited turnaround time, and identifying problems before they get the point that manufacturing or testing has to stop.

STARLIMS Advanced Analytics
For one of STARLIMS’ pharmaceutical industry clients in the Asia-Pacific region, these insights have led to increased productivity and efficiency. The customer implemented the STARLIMS platform to manage its internal QC lab testing processes. Through the use of STARLIMS the customer was able to reengineer laboratory processes and was able to make 6 FTE savings, a significant achievement. More importantly, the lab was able to significantly increase capacity. Through implementing STARLIMS as an automated LIMS, the customer was able to relocate resources in the lab and streamline their manufacturing workflow. As a result, they were able to take on new drug manufacturing streams and increase productivity and profitability, without having to increase their personnel.
With the life science industry relying heavily on outsourcing routine testing to third party service providers, STARLIMS offers a user-friendly, real-time portal for implementing testing requests, and visualizing sample and testing data. The portal saves time and minimizes the potential for manual errors in ordering or reporting. For both internal and external customers this portal is how the sample gets in the front door and how it gets reported out the back door
STARLIMS unified platform was developed organically and has kept evolving over the past 36 years, applying technologies, releasing new products, including ELN (electronic laboratory notebook), SDMS and LES (laboratory execution system) in house, and not through integration with third party products that then have to be skewed or re-engineered to fit. STARLIMS’ agile R&D development methodology allows the acceleration of the development of new products and new capabilities. It’s a ‘DevOps’ (development and operations) model whereby the innovation is driven by the customer and by market needs, and which STARLIMS can respond to rapidly through a fluid relationship between developers and IT specialists. This Agile DevOps strategy has allowed STARLIMS to enhance its service portfolio to meet tomorrow’s needs more quickly.
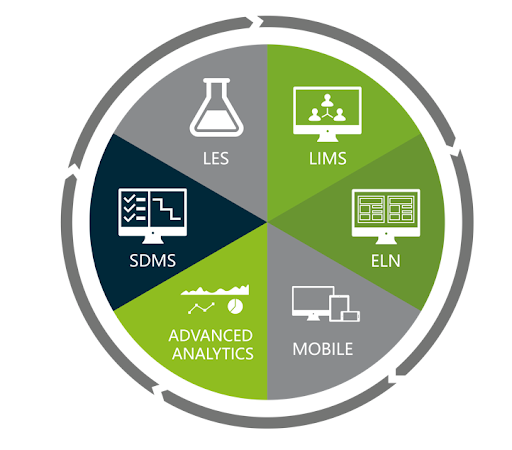
STARLIMS Integrated Solution
Organizations will base their LIMS purchasing decisions on the product meeting functionality requirements – and whether it has already been proven in the marketplace – as well as on price, but also on which provider they will feel most comfortable doing business with. Technology notwithstanding, what sets STARLIMS apart in its 36 years of evolution is its ethos of partnering with customers, not just selling products. It's a philosophy that is grounded in taking the time to learn about customers’ businesses, where that business has come from and where it is going. STARLIMS customers are customers for life. It's an added value proposition that is grounded in the customer service, support and knowhow that STARLIMS can offer. As customers’ businesses change, STARLIMS can provide them with the knowledge and expertise of how the informatics solutions can support that change.
This ongoing and close relationship with clients also helps to drive evolution of the STARLIMS suite. The team understands and predicts how the informatics landscape needs to change and speeds the development of functionality that will support current and future requirements for different industries. A quality product must come with excellent customer service and support, to win business and differentiate what may on paper look like equivalent technological capabilities. And that’s why some of STARLIMS’ longstanding clients have been with them for 30 years plus.
Engendering this relationship with customers requires teamwork, and an outlook that is focused on local, as well as global needs and expectations. STARLIMS Corporation takes pride in its people culturally as well as professionally. People are encouraged to leverage their strengths, and the executive team look at the talents of every individual and work with them to develop their roles, give them the best training and experiences at all levels.
Organic growth will continue for STARLIMS Corporation as it expands its global customer base, further develops its product suite and scales business through cloud computing. Their aim is to focus on high growth markets and high growth geographies, while serving existing and new clients across industries. As a key player in the LIMS marketplace for 36 years, STARLIMS has been actively enhancing its collaborations and development where it plays. For example, their collaboration with Allotrope Foundation, where STARLIMS helps to drive and inform the development of data standards which vendors are now incorporating into their new product offerings. Such standards will make data more accessible, searchable, transferrable, and insightful. The goal is to promote that ability for data exchange and utility, across laboratories and organizations. It’s more than a solution.
To Know More , Please email STARLIMS India: AISalesIndia@abbott.com











