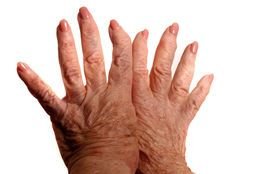
Singapore Health Sciences Authority (HSA) nod for Aslan Pharma rheumatoid arthritis drug ASLAN003
Singapore: Aslan Pharmaceuticals received approval by the Singapore Health Sciences Authority (HSA) for its Clinical Trial Certificate Application for ASLAN003, which is a novel oral small molecule that has the potential to be a safer and more effective therapy than current treatments for rheumatoid arthritis (RA).
Aslan will now begin a multiple ascending dose phase I study in Singapore to assess the safety profile, tolerability and pharmacokinetics of ASLAN003. Aslan currently has three drugs in clinical development, and is focused on oncology and inflammation diseases.
Dr Mark McHale, chief scientific officer, Aslan, said that, "RA is a global disease, and in Singapore affects around 45,000 people. While there are several therapies available for rheumatoid arthritis today, physicians and patients continue to look for safer, more effective and more accessible therapies. The quality and experience of clinical centers in Singapore, such as Changi General Hospital, make this an ideal place for these important studies."




