
The national government has supported the establishment of clinical research centers in hospitals and the Taiwan FDA (TFDA) has added a new fast-track review process for global and regional trials.

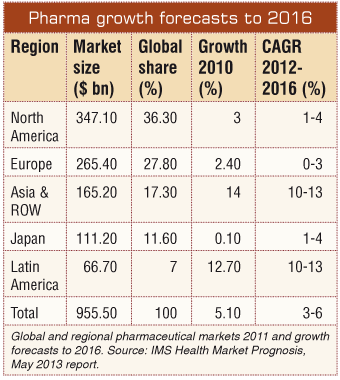
The loss of patents on the last generation of blockbuster products has forced multinational pharmaceutical companies to focus their attention not only on creating new products but also on developing new markets. As a result there has been a shift towards emerging markets, whose share of global revenues is forecast to rise from 18 percent in 2010 to 28 percent by 2015, according to IMS: Market Prognosis, April 2011 report. Among the emerging pharmaceutical markets, Asia - with its vast potential for efficient clinical development and enhanced revenues - continues to grow at double-digit rates. This interest in Asia Pacific markets is clearly reflected in an extraordinary growth of research and development (R&D) investment which has been reported to have increased more than 20-fold between 2000 and 2010.
Not surprisingly, this increase in research and development expenditure is reflected in an upturn in clinical development activity in the region. Asia Pacific countries offer a number of potential advantages for clinical studies, including vast patient populations, spectrum of disease, fast recruitment and excellent retention, well-trained investigators, high-quality sites and significant cost savings in comparison with the US and EU countries.
In 2009, Frost & Sullivan predicted that the Asian clinical research market would grow at a CAGR of 20 percent between 2010 and 2015. In particular, the report forecast continued strong growth for clinical research markets in China, Korea and Taiwan, according to The Rising Dominance of the Asian CRO Market. Frost & Sullivan, 2010, report. Data from the Taiwan FDA shows that clinical trial activity in Taiwan has been increasing steadily over the past eight years.
The national government has supported the establishment of clinical research centers in hospitals and the Taiwan FDA (TFDA) has added a new fast-track review process for global and regional trials. Provided that a multinational clinical trial protocol has been approved by one of 10 reference countries, then the TFDA will undertake only an administrative review without requiring technical evaluation from the Centre for Drug Evaluation. Many international and local biopharmaceutical companies are initiating an increasing number of clinical trials in Taiwan, either in the form of local studies or as part of regional or global international multi-center studies. As in other markets, many sponsors outsource some or all of their Taiwan clinical studies to contract research organization (CRO).
In this regard, there are significant advantages in partnering with a CRO which has specialist experience of the medical, political, regulatory and cultural environment of Taiwan and the Asia Pacific region, regarding regulatory consultancy, strategy and planning, and advice, protocol design, development and communication, feasibility and site selection, patient recruitment and retention, pharmacovigilance, DSMB (safety monitoring and signal detection), and data management and statistical analysis (audit trail management).
Proactive expert support across these study areas can help to ensure that sponsors reap the multiple benefits associated with running trials in Taiwan while reducing the risk of delays and complications. Taiwan is a growing location for pharmaceutical studies and this will herald continuous challenges and opportunities for clinical development. In order to rise to these challenges, there is enormous potential for ethical and ambitious biopharmaceutical companies working in partnership with regional clinical research organizations that combine local expertise with the highest global standards of quality and service.




