Photo Credit: Colder Products Company (CPC)
The FDA’s approvals of more cell and gene therapies (CGTs) are cause for celebration — but the marathon is not done. Attention has quickly turned to conquer the challenges of commercial-scale manufacturing for these promising therapies. Many of the current CGT manufacturing processes used to supply clinical trials are inadequate for commercial scale. Fortunately, some learning can be adopted from bioprocessing, a process with similar needs.
Understanding the Needs of CGT Manufacturing
The pipeline of CGTs is impressive. In addition to the recent FDA-approved therapies, over 400 therapies are in preclinical to Phase 3 development and approximately 1,700 clinical studies are underway globally.
Recent FDA approval of CAR T cell (chimeric antigen receptor —T cells)-based therapies has heightened interest and investment in CGTs. Autologous cell therapies (i.e., same donor and recipient) grew 65 percent from 2016 to 2017. It is now time to tackle the challenges of sustainable and cost-effective commercial manufacturing for these emerging therapies.
Single-use technologies (SUTs) are expected to play a huge role in CGT commercialization. CGTs have many of the same needs as biopharmaceutical manufacturing, where SUTs have proven to effectively meet the needs at the commercial scale.
While SUTs are used to manufacture CGTs for clinical trials today, there are limitations. Many of those SUTs cannot be transferred from lab scale into commercial scale for a variety of reasons, including extractable, leachables, supply chain security, reproducibility, and scalability. The industry needs to overcome these issues and develop solutions in the near future to meet the pace of CGT development and expected demand.
Comparing the Manufacturing Processes
Biologics and CGT manufacturing processes have some similarities and some differences.
Therefore, the production tools and techniques used to make these different therapies have some similarities and some differences. Similarities are where the transfer of learning can take place.
Biopharmaceutical drugs utilize SUTs at all stages in the development and manufacture, from bench-scale research through all phases of clinical testing and into commercial manufacturing. SUTs are used for the development and production of both large and small molecule drug products. The wide range of processing technologies are supplied as either discrete components or more often as pre-validated, pre-sterilized single-use systems ready to use once opened. SUT adoption provides many well-documented benefits for commercial operation, including:
Similarly, for cell therapies and personalized medicines, SUTs are widely used and accepted for development and production. The reasons for using SUTs for CGTs are strikingly similar to those for bioprocessing — cost, speed, and sterility.
Contrasting the Two Manufacturing Processes
Scale is one significant difference between bioprocessing and CGT processing. Bioprocessing scales are larger than those used in the development and production of autologous cell therapy products. The smaller scale of autologous products requires smaller equipment.
The type of SUTs used is the second important difference. For biopharmaceuticals, SUTs include filters, cell culture systems, mixing systems, storage vessels, tubing, sensors, valves, sampling systems, and connectors.
For cell therapies, single-use systems are traditionally employed for clinical and R&D uses for such devices as pipettes, blood collection bags, and T-flasks. The use of these products will continue but is being supplemented with the expansion of SUTs to include collection sets, fluid transfer sets, small-volume cell culture systems, and specifically with the widespread utilization of single-use bags and bag assemblies for media, washing, rinsing, cell harvest, waste collection, and even cryopreservation.
Identifying the Gaps of Cell Therapy Manufacturing
Currently, the cell therapy industry is composed of a wide range of disciplines, experiences, technologies, and applications. Different groups working on the research and development of therapies bring different levels of knowledge and perceived requirements to the discussions on SUTs and systems.
Many of the technologies and equipment currently used for CGT were originally designed for other purposes and have been adapted for use in cell therapy manufacturing. It is unlikely that all the technologies and systems used today will have either the appropriate technology or the required documentation to support the manufacturing requirements for late-stage clinical phases and commercial production.
In order for a manufacturing process to move from development into commercial manufacturing, the following issues need to be addressed:
Mapping the Future of SUT in Cell Manufacturing
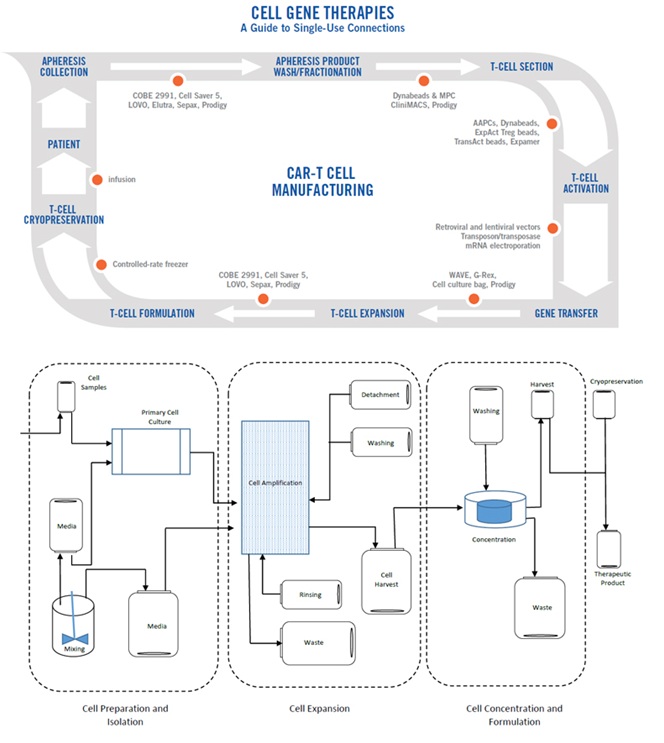
Cell therapies are developed using a variety of processes today. The process is dependent on many factors, including cell source, processing requirements, methods of cell harvesting, cell selection, cell washing, cell expansion, gene modification, and gene transfer. The process steps for CAR-T development are shown in Figure 1 below in the outer circle. Examples of technologies that can be used for each process step are also shown below in the inner circle. Some of the technologies listed are very similar to those used in large-scale bioprocess manufacturing applications.
As more cell therapy products enter both the clinical trial pipeline and commercial production, it is important to identify the benefits SUTs can offer for cell therapy developers and manufacturers and to implement SUT where appropriate. Equally important, the industry needs to define the knowledge gaps, the potential pitfalls of SUTs, and the path forward for obtaining the information to make decisions.
The consequences of not planning ahead are significant. Imagine reaching a late-stage clinical trial only to learn a change to a single-use manufacturing component has to be made. This could result from product discontinuation, lack of scalability, supply chain issues, or validation issues. The impact of having to make a process change could be significant delays in time to market, increased costs for revalidation, and potentially additional regulatory investigation of the other technologies used in the manufacturing process.
Evaluating the Cost of Current CGT Manufacturing
In a recent presentation, the cost of manufacturing a single therapy using current labor-intensive processes was provided based on the following scenario:
The estimated operating cost is $400 million annually, including a dedicated 70,000-square-foot facility and a workforce of approximately 3,700 trained technicians and scientists.
However, because of the manual processes, the risk of process failure would be high, thereby significantly reducing the number of patients who could be treated and substantially increasing the cost of each effective therapeutic treatment. This is clearly not a long-term, sustainable, cost-effective option.
Learning About SUTs
Cell therapy manufacturers should be aware of the wide range of SUTs available. The sheer number of SUTs, even within a component type such as storage bags, connectors, or cell culture systems, can be daunting. New users should anticipate a steep learning curve. However, a tremendous amount of application, technical, and processing information about SUTs exists in the public domain, from single-use manufacturers and from industry organizations.
The scope of this paper does not permit exploration of all SUT products, but instead focuses on one of the most important but often overlooked decisions when developing a single-use-based process — the right connection technology.
Connection technology is what brings all the pieces of the single-use jigsaw together. The right connection technology can allow the aseptic connection of single-use to non-single-use steps in the process while maintaining a fully closed system. The wrong connection technology can have serious implications on the scalability, reproducibility, and security of the process.
Understanding Single-Use Fluid Path Connections
Fluid connection technologies fall into two basic categories based on how the connection is achieved:
To select the appropriate connection technology for an application, it is essential to understand the critical technical differences both between the main technology types and between subgroups within each technology group, plus the operational impacts those differences would have on the process and the benefits of each technology.
How many connections will be required within a cell therapy process? While the answer is totally processed -, product use-, and scalability-dependent, Figure 2 shows an example of a cell therapy process based closely on the CAR-T process from Figure 1. It is composed of flexible film-based SUTs. In this proposed system, each bag in the process could be 50 mL to 5 L in size depending on the application and volume of liquids handled at each step. Figure 2 illustrates the number and location of connections required as part of this process.
Determining the Connection Technology Type
An important factor in determining the appropriate connection technology is the design of the processing system. Will it be open or closed? Typically, the location of the process development stage has a significant impact on the design of a processing system and the components used (Table 1).
In a clinical or academic laboratory, product development looks very different than in a biopharmaceutical company. Not only are the technologies different, but the longer-term objectives are different as well. When process development is done in a biopharma company, decisions are often made with a longer-term view that has commercialization as the ultimate objective.
| SETTING | COMPONENTS | PROCESS STEPS | PROCESS | SYSTEM |
| Laboratory | Tissue culture Dish, plates, flasks, vials | Transfer by pipettes | Manual | Open components and processing |
| Clinical | Syringes, Processing and storage bags, transfer sets | Luer connectors, sterile docking, tube welders | Manual with some automation | Closed connectors and processing |
| Biopharmaceutical Company | Rocker bags, culture bags, roller bottles, bioreactors | MPC MPX connectors, aseptic connectors, tube welders | Manual with a high level of automation | fully closed system |
SUTs, including easy-to-use aseptic connectors, can address the requirement of greater process robustness and reliability. While SUTs are being increasingly adopted in recombinant manufacturing processes, their adoption in cell therapy bioprocessing is essential due to sterility concerns. In contrast to the well-established automated processes used in recombinant therapy production, the poor automation, labor-intensive, and open nature of cell therapy manufacturing make it more prone to operator variability and contamination risks.
In its Technology Roadmap to 2025, the National Cell Manufacturing Consortium clearly identified in the section on Standardization and Regulatory Support that establishing standards with the appropriate regulatory authorities is required for both open and closed systems. The type of system required and the environment in which each process step and connections for each step will be undertaken determine the type of connection technology that should be used.




