Q. Can you briefly explain this new blood testing technology and how is it different from the more conventional tests that path labs do?
The cobas m 511 represents a new era of haematology testing and is the most dramatic innovation the industry has seen in decades. What makes the cobas m 511 analyser truly unique is that it is the first, fully integrated digital analyser which combines three conventional haematology systems into one, compact platform.
It uses cutting-edge Bloodhound technology and automatically prints, stains, scans and counts blood cells, producing qualitative and quantitative results in just six minutes. When I started developing this technology back in 2004, the purpose was to create a system that could overcome the limitations of conventional haematology testing such as the need for multiple systems, the number of human touchpoints which increases the risk for contamination and error, the time required for the test which could range from 15 to 30 minutes and most of all the inconsistency in generating reproducible and standardised morphology results.
The cobas m 511 addresses all of these challenges. The system generates standardised, accurate and replicable results which can help to ensure greater consistency in clinical decisions and take uncertainty out of decision-making.
It uses a much smaller volume of blood just 30 microlitres, which is a third less than conventional methods. In the fields of paediatrics, this is ground breaking. Taking samples from premature babies or infants with illnesses is risky and can have severe health consequences. Bloodhound® eliminates the need for 'top up' blood transfusions which doctors need to perform to replace the amount of blood lost through current testing.
Conventionally, lab technicians need to prepare, stain and analyse the blood samples using various instruments. The cobas m 511 on the other hand prints a monolayer onto the slide, stains with an improved method for further analysis of the morphology and enables classification of cells displayed on a Viewing Station flagging any abnormal cells. This is why I called it Bloodhound, as it tracks down and presents the abnormal blood cells.
Even if nothing abnormal is flagged in the blood sample, the images are saved digitally for review. These digital slide images can be archived and emailed so that results can be shared and reviewed by experts remotely, from anywhere the world. This isn’t the case with conventional flow based methods, and is a significant advantage for medical professionals.
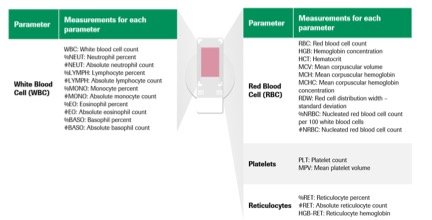
Q. What is the scope of the blood tests that can be done using this? More specifically, are there any specific tests or results that cannot be obtained using this new approach / invention?
The technology counts, analyses morphology and classifies every cell in the viewing area to provide a complete blood count (CBC) and 5-part differential and reticulocyte count.
Haematologists still have the option of looking at slides under their microscopes, but as the technology provides cell-by-cell images it may eliminate the need for microscopic review.
Q. In terms of the speed of obtaining results and the accuracy of them, how do they compare to blood tests done by path labs presently?
The technology generates results in just six minutes. This is much faster than conventional lab methods which can take around 15-30 minutes – an 80% reduction in turnaround time. Over the course of a day, week or year this can make a huge difference for laboratories and the technicians there as it means they can better focus their resources and expertise.
More importantly, the technology produces standardized and replicable results so that they can be easily and accurately interpreted. This takes any uncertainty out of decision-making in terms of diagnosis or treatment options. The system is also fully automated, meaning fewer human touchpoints, no manual steps and therefore the opportunity to minimise the risk of human error and ensure greater accuracy.
Q. Are you seeing good adoption for this invention? Any concerns or impediments that are likely to slow it - in terms of costs, accuracy, learning curve to use this, etc? Any other reason that may cause resistance in this becoming mainstream quickly?
We have already successfully launched the system in Pakistan, with Hong Kong and Japan planned for early next year. We are also expecting to launch throughout Asia Pacific in 2018. Timings always vary between countries due to the different registration processes across the region.
The cobas m 511 is a significant advancement in the field of haematology and we are confident that the laboratories in the region and around the world will benefit tremendously from this system. In Europe, the cobas m 511 is already available and we are currently assessing the technology. With laboratories, including those in Asia, facing increasing medical need and a shortage of haematology experts, we think our system will add value and greater efficiency.
The technology doesn’t replace humans in the laboratory, instead it frees up time and resources for the technician to better utilise their expertise in other aspects of their work in the lab. The cobas m 511 helps lab technicians concentrate on what really matters: finding and classifying abnormal cells within a patient sample which helps doctors in making quick and accurate diagnosis. The ultimate beneficiary of this innovation is the patient.
Q. Are there any other related diagnostic inventions (in the pipeline or already available) that can potentially complement and/or revolutionize the way pathology tests are conducted?
The cobas m 511 is the first innovative product from Roche in the field of haematology, and the world’s first and only 3-in-1 integrated haematology system.
We are working to make the Bloodhound technology even more sensitive to rare abnormal cells that might be present in a patient’s blood. This would allow for earlier detection of, for example, reoccurring cancers that might otherwise be missed, especially cancer of the blood.
The Bloodhound technology currently provides measurements related to the size and shape of the cells. So, we are able to give more information to the laboratory than you can with any other system. Now we are also looking for parasites in the red blood cells which will be a big advantage, especially in Asia Pacific where prevalence rates of malaria and other blood-borne diseases are still high.




