Image Credit: prnewswire
BD (Becton, Dickinson and Company), a leading global medical technology company, has announced that U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for its rapid antigen test to be used for SARS-CoV-2 screening through serial testing of asymptomatic individuals.
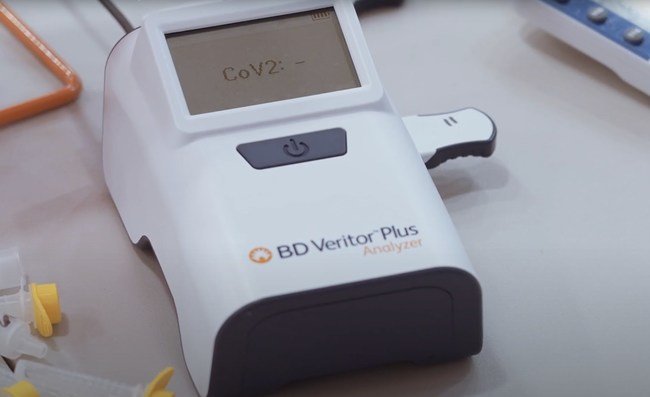
The BD Veritor™ Plus System supports this approach in everyday locations such as schools and businesses, along with serial testing in other situations, such as athletes and teams to ensure safe games and competitions.
The EUA for the BD Veritor™ Plus System includes SARS-CoV-2 screening through serial testing of asymptomatic individuals when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests. Serial testing on the BD Veritor™ Plus System can be performed in any setting with a CLIA certificate of waiver.
The BD Veritor™ Plus System for Rapid Detection of SARS-CoV-2 is intended for the qualitative detection of SARS-CoV-2 nucleocapsid antigens in direct anterior nasal swabs from individuals who are either suspected of COVID-19 by their health care provider within the first five days of the onset of symptoms, or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.




