Singapore based molecular diagnostics company Lucence and the Agency for Science, Technology and Research (A*STAR) have announced study results indicating that the reagent used in Lucence’s SAFERSample saliva collection kit inactivates SARS-CoV-2 within 45 seconds of sample collection.
This proprietary reagent also stabilizes viral RNA at room temperature for up to one week. Together, these properties enable accessible, non-invasive, and safe sample collection via saliva, which is critical to scaling cost-effective testing worldwide. The reagent used in Lucence’s SAFER Sample kit was invented at A*STAR’s Institute of Bioengineering and Nanotechnology.
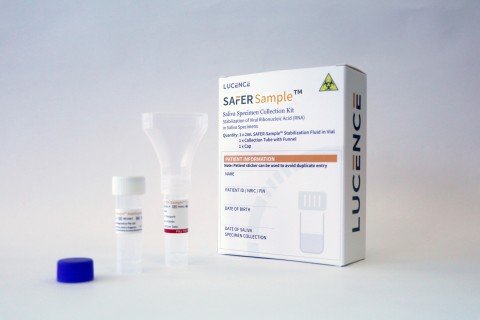
The SAFER Sample Collection Kit is registered as a Class A medical device with the Health Sciences Authority of Singapore and is an easy-to-use saliva collection kit that comes with a bottle of stabilization fluid to be mixed with the sample at the point of collection, keeping viral RNA stable at room temperature for up to one week.
While conventional viral transport media requires cold-chain transport to prevent sample degradation, which can drive up associated costs and limit testing geography, SAFER Sample’s proprietary media means samples can be transported to a lab without the need for chilling.
Studies with clinical partners are ongoing to further evaluate the performance of the SAFER Sample kit.




