image credit- prnewswire
Taiwan Main Orthopaedic Biotechnology (Surglasses), a medtech startup based in Taiwan, has announced that Caduceus S AR Spine Navigation System has received 510(k) clearance from the US Food and Drug Administration and the startup is ready to launch its products into the US market in the first quarter of 2023.
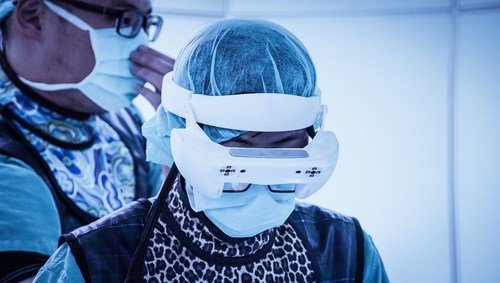
Caduceus S is an augmented reality (AR) spine navigation system that will require only few-shots of C-arm images to equip the surgeons with AR and pre-surgical planning with trajectories for spine surgery.
The system is designed to allow surgeons to superimpose the 3D spinal anatomy on a patient during surgical procedures. It is equipped with multiple trackers and displays preoperative planned trajectories via 3D AR head mounted display.
Caduceus S has received its Taiwan FDA (TFDA) clearance in August 2022 and is being used into the surgical procedures in Taiwan since then. Results from using the Caduceus S revealed 98.2% accuracy using the Heary (thoracic) and Gertzbein (lumbar) scales. The company launched the first-in-human surgery of its Caduceus S Spine system in October 2022 in Taiwan for a scoliosis case with 58。 Curvature.




