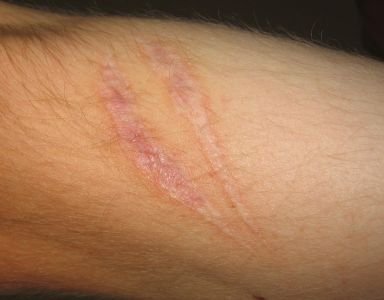
Strataderm is a rapidly drying, non-sticky, transparent, silicone gel formulation for the treatment of scars
Singapore: China-based Luqa Pharmaceuticals has launched its first product in China, Strataderm Gel, an innovative silicone gel scar treatment that has been approved by the State Food and Drug Administration (SFDA). Strataderm is a rapidly drying topical formulation that helps prevent excessive scarring following surgery.
Founded in 2010, Luqa is a private company backed by healthcare focused European private investors. It is headquartered in Hong Kong with an office in Shanghai and a focus on the China market. Luqa in-licensed China rights to the product from Stratpharma of Switzerland in August 2012. Luqa specializes in topical products, making Strataderm a natural fit. It expects to launch an additional product in China before the end of 2013.
Strataderm is a rapidly drying, non-sticky, transparent, silicone gel formulation for the treatment of scars, both old and new. It has been developed using a new silicone polymer technology that is self drying without the use of Silicone Dioxide. Silicone gel is the only non-invasive scar treatment option for which evidence based recommendations have been made. The International Clinical Recommendations on Scar Management states, "Silicone gel should be used as first line therapy in the initial management of scars and in the prevention of hypertrophic scars and keloids".
Up to 94 percent of Chinese patients develop hypertrophic scars following median sternotomy incision and 41 percent of women develop a hypertrophic scar within 12 weeks of caesarean delivery. Strataderm is one of the few clinically proven products for scar therapy that are currently available in China.




