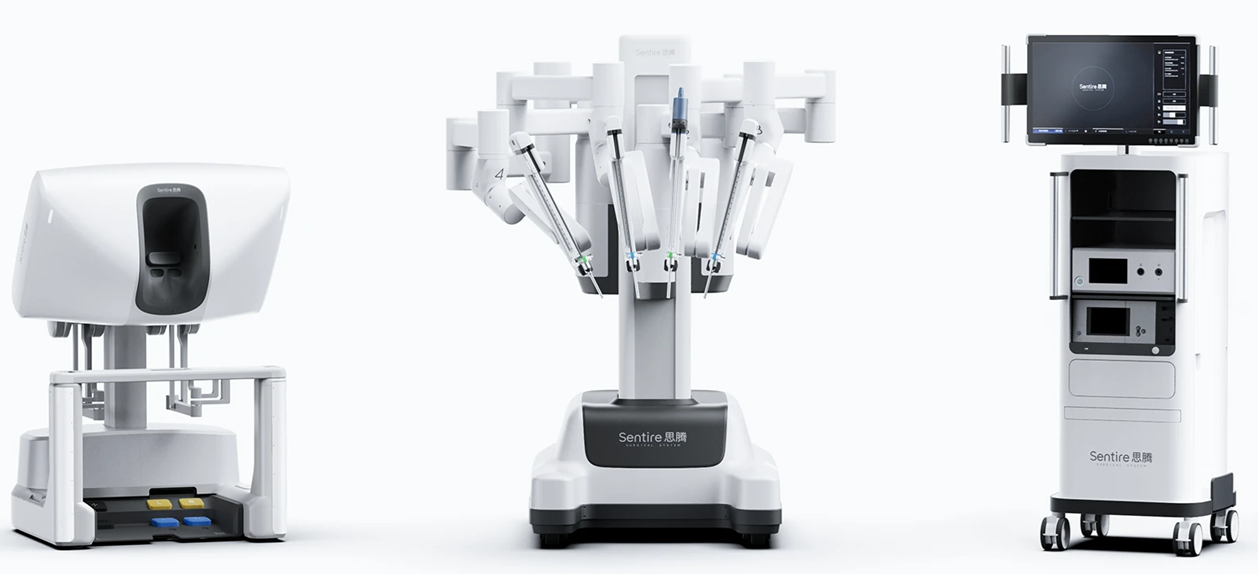
Cornerstone Robotics has announced the launch of its first formal clinical investigation of its Hong Kong-developed surgical robotic system at Portsmouth Hospitals University NHS Trust (PHU), Portsmouth, UK.
The Sentire® Endoscopic Surgical System (C1000)—developed by Hong Kong-based Cornerstone Robotics Limited—is now being evaluated at PHU’s Queen Alexandra Hospital in Portsmouth, UK. This groundbreaking initiative represents the first clinical deployment of the Sentire® System in the UK and Europe, a major milestone in international clinical collaboration that marks the start of Cornerstone’s preparations for commercial activities in the region.
Led by Professor Jim Khan, PHU’s surgical team kicked off the start of the clinical trial with three successful colorectal procedures using the Sentire® system. The clinical investigation is focused on assessing the safety and efficacy of the Sentire® system for performing colorectal and urological procedures within European populations—expanding upon previous clinical data generated in Asia. The trial follows international standards for device evaluation, marking a significant regulatory and clinical first for a Chinese robotic platform.
The Sentire® Endoscopic Surgical System combines clinical workflows with integrated engineering, software, and imaging technologies to support surgeons in performing intricate minimally invasive procedures. Early clinical feedback suggests that these features may contribute to streamlined workflows for surgical teams and improved outcomes for patients.
This collaboration reinforces PHU’s position at the forefront of surgical robotics. PHU is the first in the UK to operate a dedicated robotic-assisted day surgery programme and has performed over 5,000 robotic procedures across colorectal, urological, upper gastrointestinal, and gynaecological specialities—offering a scalable model for NHS adoption of advanced surgical technology




