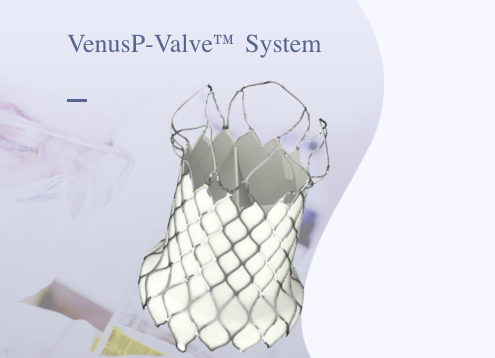
VenusP-Valve, Venus Medtech's in-house developed innovative transcatheter pulmonic valve replacement (TPVR) system, received CE marking under the Medical Devices Regulation (MDR) to be marketed in Europe. Designed to treat patients with moderate to severe pulmonary regurgitation with or without right ventricular outflow tract (RVOT) stenosis, VenusP-Valve is the first Chinese-made artificial heart valve approved in Europe, marking a new milestone in the global presence of China's innovative medical devices.
As the first self-expanding TPVR product approved for marketing in Europe, VenusP-Valve carries remarkable clinical value. Uniquely designed with both flared ends, the product provides stable anchoring and easy delivery, with no need for pre-stenting before the procedure. Available in a variety of specifications with extensive applicability, the product is able to meet the needs of 85% of patients.
Following its first clinical implantation in 2013 by Academician Ge Junbo, Director of Cardiology at Zhongshan Hospital, Fudan University, VenusP-Valve has been used in nearly 300 cases for humanitarian reasons, spanning more than 20 countries and regions in Asia, Europe, North America, and South America. In March 2021, VenusP-Valve received special use authorization from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for use in designated medical institutions.




