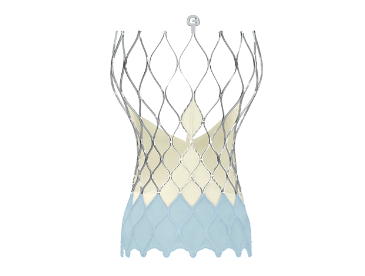
image credit- medtronic
Medtronic has announced that the National Medical Products Administration (NMPA) has approved the CoreValve™ Evolut™ PRO TAVR system for the treatment of severe aortic stenosis (AS) for symptomatic patients in China who are at high or extreme risk for open heart surgery.
As the first Medtronic self-expanding TAVR system approved in China, the Evolut PRO system approval is based on clinical data from more than 32,000 patients, which showed high survival, low rates of stroke, minimal paravalvular leak (PVL) and excellent hemodynamics (blood flow). Full commercial launch is anticipated in early calendar year 2022.
The Evolut PRO TAVR system is the next generation of the clinically proven supra-annular CoreValve Evolut™ R system that provides industry-leading hemodynamic performance. The recapturable and repositionable Evolut PRO valve features a self-expanding nitinol frame with an outer wrap that adds surface area contact between the valve and the native aortic annulus to further advance valve sealing performance.




