The Xu lab at Shanghai University's iHuman Institute has deciphered the high-resolution crystal structure of the first human Frizzled receptor. The Frizzled receptors (FZDs) serve as a gatekeeper protein to regulate the basic Wnt signaling pathway in embryonic development and tumorigenesis.
Present results demonstrating the unique structure of the Frizzled-4 receptor in a ligandless state and providing an explanation for the long-standing barriers to identifying potent ligands for this family of receptors will, however, be found in basic research and treatment approaches, which can lead to important new discoveries in medicines, benefit equally.
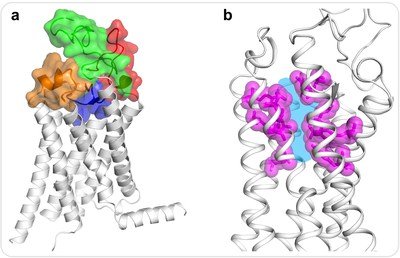
"To understand why so far no one has been able to develop good ancillary ligands or drug molecules for FZDs, we have augmented the intact structure of the transmembrane domain of the Frizzled4 receptor (FZD4) at a resolution of 2.4 angstroms," Fei Xu said, Assistant Professor at iHuman Institute and ShanghaiTech University School of Life Science and Technology, and co-author of the study.
It was a surprise to the authors to observe that the common binding site of the orthosteric ligand is very narrow and hydrophilic, making it difficult for small molecules to penetrate or bind. "These results allow us to better understand the properties of FZD ligands and signaling pathways, and show that they have to worry about the ligand design for this molecule pocket, which might be based on this crystal structure," Fei Xu said.
"The team spent four years developing a more stable human FZD4 protein that can be used to determine the structure even without ligands, and it has screened hundreds of constructions, optimized purification procedures, and tried thousands of conditions for crystallization "said Shifan Yang , postdoctoral fellow at iHuman Institute and lead author of the article.
In addition to the discovery of a vacant molecular bag, this work also provides insights into a unique mechanism for activating the Frizzled family. "Such a remarkable structure provides a more accurate basis for directing Frizzled drug discovery efforts in the right direction," said Raymond Stevens, director of the iHuman Institute at ShanghaiTech University.
The other co-authors of the article are from Shanghai Tech University, the Van Andel Research Institute and the University of Southern California.




