Australia's Vaxxas secures TGA approval to manufacture high-density microarray patch (HD-MAP)
December 15, 2025 | Monday | News
Enables GMP manufacturing to accelerate clinical trials and transform global vaccine delivery.
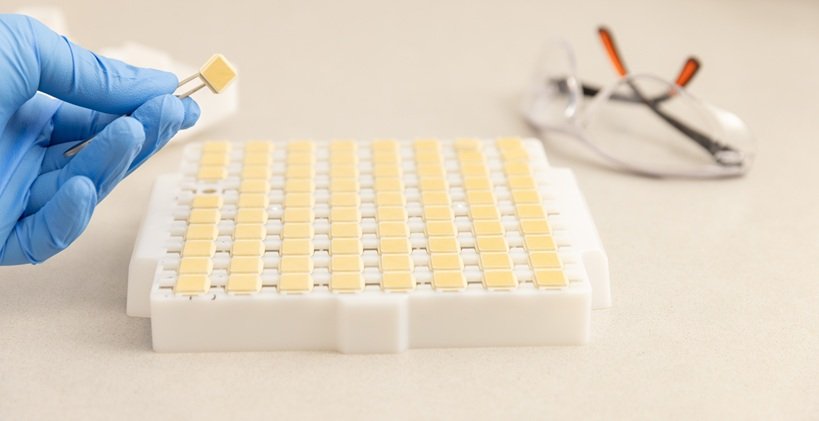
Image credit: Vaxxas
Australian biotechnology pioneer Vaxxas Pty Ltd, has been granted a licence by the Therapeutic Goods Administration (TGA) to manufacture the Company’s proprietary high-density microarray patch (HD-MAP) for clinical trials at its state-of-the-art biomedical facility in Brisbane.
The TGA licence strengthens Vaxxas’ leadership in next-generation vaccine delivery and follows installation of the Company’s first robotic lines for aseptic (sterile) manufacture.
Designed to deliver all major vaccine types to the skin using a simple, easy-to-use applicator, the HD-MAP has the potential to be a universal delivery solution for vaccine self-administration.
The manufacturing licence for aseptic (sterile) production includes principles and procedures to ensure the vaccines are of the necessary high quality, as defined by the TGA and Good Manufacturing Practice (GMP) standards.
Vaxxas Chair Sarah Meibusch said, “The TGA manufacturing licence marks a significant milestone for Vaxxas as we progress toward commercialising our HD-MAP technology. By reducing cold-chain requirements and enabling self-administration, this innovation addresses key barriers to vaccine access and uptake worldwide.”
Vaxxas Chief Technology Officer Dr Angus Forster added, “This licence unlocks the way forward for Vaxxas to continue developing our world-leading HD-MAP technology. We contribute to Queensland’s growing biotech sector and to advance sovereign manufacturing capabilities that translate cutting-edge research to address real-world health solutions.”





