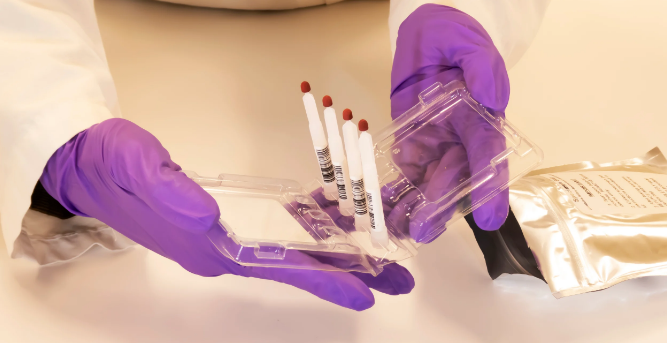
Trajan Scientific and Medical, a leading provider of analytical and life sciences products and solutions, has announced the new registration of its Neoteryx remote blood microsampling Mitra devices and Mitra Sample Collection Kits under the new manufacturing label.
The Mitra is registered with the Therapeutic Goods Administration (TGA) in Australia as a Class I in vitro diagnostic (IVD) medical device and the Mitra Kit is registered as a Class II medical device for home use.
The updated regulatory compliance expands the use of Trajan’s Mitra microsampling products in clinical settings, as well as home-based sample collection to support telehealth and personalised medicine. Doctors and research scientists seeking to boost clinical trial participation through remote sampling in rural communities, among non-traditional participants, and patients with limited access to clinical sites can simply ship the sample collection kits to these populations for self-guided specimen collection.
Mitra provides a major benefit to patients participating in clinical studies that fear needles or are inconvenienced by the requirements of a clinic-based blood draw. Following the instructions for use and a quick finger-stick, virtually anyone can use a Mitra device to collect a high-quality blood sample for accurate lab testing.
As published in the literature, specimens collected with Mitra Kits have been used for detection of inflammatory biomarkers, PSA for prostate cancer screenings, antibodies against SARS-CoV-2, and more.




