Image Credit: Abbott
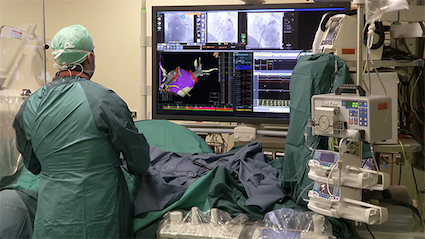
Abbott has received CE Mark and approval in Australia for its new EnSite X EP System, and is launching the system throughout Europe and Australia. The EnSite X System is the only system that offers the option to navigate the cardiac anatomy two different ways on one platform.
Abbott sought physician feedback to develop the EnSite X System to meet current needs as well as address emerging opportunities during cardiac ablation procedures. The new cardiac mapping platform builds upon the company’s electrophysiology portfolio and is designed to improve how physicians deliver ablation therapy to treat abnormal heart rhythms.
The EnSite X System has advanced imaging capabilities that allow for the creation of a three-dimensional (3D) model of the patient’s cardiac anatomy in real-time, allowing physicians to clearly see areas of the heart that need ablation treatment. Physicians can choose traditional impedance monitoring (using mechanical activity of the heart) or electromagnetic technology, which allows precise location of Abbott's sensor-enabled catheters during treatment.
"The new EnSite X System will fundamentally change how physicians approach longer, more complex ablation procedures as a result of its improved stability, faster mapping and better model visualization," said Prof. Paolo Della Bella, Head of the Department of Cardiac Electrophysiology and Arrhythmology at IRCCS San Raffaele Hospital, Milan, Italy. "In my first cases with the system it’s been apparent that the system is an important step forward in terms of technological capabilities and also truly helps improve my clinical analysis by protecting for patient movements.”
The various aspects of the EnSite X System are designed to work in harmony and allow ongoing updates as technology advances. The new mapping system is among a series of product-focused activities from Abbott designed to improve patient care and meet the needs of electrophysiologists around the world.
The company also recently secured U.S. Food and Drug Administration (FDA) and CE Mark approval for the EnSite LiveView Dynamic Display, which allows data from the Advisor HD Grid mapping catheter, Sensor Enabled to be visualized in real-time during cardiac ablation procedures.




