Image Caption: Affinity’s FACS (cell sorter) identifying COVID-19 antibody clones
Melbourne-based Affinity Biosciences Pty Ltd (Affinity) has lodged a pre-publication submission to the scientific journal, ‘mAbs’, detailing its results and observations relating to the discovery of potent antibodies against COVID-19.
This can be found at the link, 'Antibodies that potently inhibit or enhance SARS-CoV-2 spike protein-ACE2 interaction isolated from synthetic single-chain antibody libraries’.
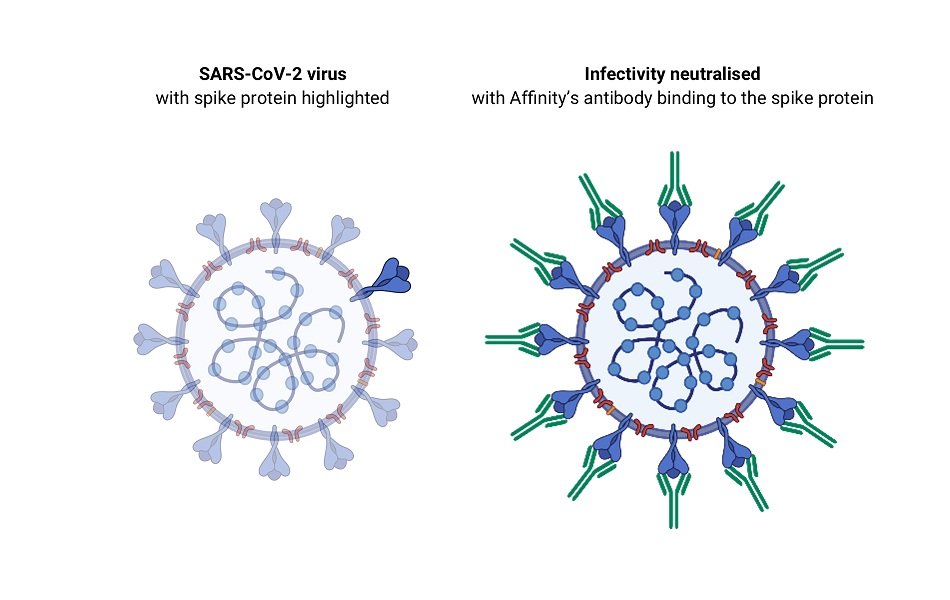
Affinity’s lead antibodies have been shown to be capable of completely neutralising SARS-CoV-2, the virus that causes COVID-19, and are amongst the most potent reported to date. The virus neutralisation data generated under contract by The Peter Doherty Institute for Infection and Immunity in Melbourne have been previously announced by Affinity.
Affinity is now aiming to have its COVID-19 antibody therapy available to the public as fast as possible. Affinity has requested proposals for the manufacture of their antibody for clinical trials from Australian and overseas contract manufacturing organisations and is in discussions with regulatory consultants to establish an expedited path for manufacturing, trials, and approval of the antibody therapy.
Affinity’s CEO, Dr Peter Smith, said that material for clinical trials can be manufactured in as little as three to four months, which will provide an opportunity to initiate clinical trials before the end of the year.
Securing funding for manufacturing will also ensure local supply. This will be especially important if access to overseas supplies of vaccines or therapeutics are limited or products take longer to develop than expected.
Vaccines generally work by inducing the production of antibodies that neutralise the virus. Affinity’s antibodies, when injected into patients, should achieve the same effect of neutralising the virus for a short period, likely to be measured in weeks. It will therefore be potentially useful as a short-term therapy or to protect people in high risk situations such as healthcare workers or people in direct contact with COVID patients.
Another observation detailed in the pre-publication submission is the discovery of antibodies that increase binding of the virus spike-protein to human ACE2. This observation may be important for vaccine development because if a vaccine induces similar antibodies, it could potentially reduce its effectiveness. Affinity is now investigating how these antibodies function to inform vaccine design moving forward.
Affinity is currently engaging with government agencies and other sources of funding to enable the start of manufacturing and clinical trials for their antibody therapy.




