Good news for cystic fibrosis patients - FDA approves Novartis TOBI Podhaler
Singapore: US Food and Drug Administration (FDA) has approved Novartis' TOBI Podhaler (tobramycin inhalation powder) 28 mg per capsule for the management of cystic fibrosis (CF) patients with Pseudomonas aeruginosa (Pa) bacteria in the lungs. Pa is the leading cause of loss of lung function in CF patients. It is not known if TOBI Podhaler is safe and effective in patients under six years of age, in those with lung function outside of a certain range, or in those whose lungs contain bacteria called Burkholderia cepacia.
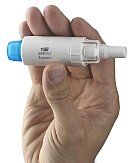
TOBI Podhaler is a new, non-nebulized formulation and delivery system of tobramycin, the same active ingredient as in TOBI (tobramycin inhalation solution, USP) 300 mg/5 mL, which has been on the market for approximately 15 years. TOBI Podhaler is the first and only FDA-approved dry powder inhaled antibacterial for Pa in the US. It delivers tobramycin into the patient's lungs via a pocket-sized dry powder inhaler and offers better portability than TOBI, which is administered using a nebulizer. In a Phase III study, TOBI Podhaler shortened administration time for patients by approximately 70% compared to TOBI, saving about 13 hours per treatment cycle. This does not include the time saved on setting up and maintaining the nebulizer and compressor.
TOBI Podhaler does not need to be stored in a refrigerator and, unlike nebulized Pa treatments, does not require a power source to operate the delivery device. While the nebulizer used to administer TOBI must be disinfected in boiling water for 10 minutes every other treatment day, the disposable Podhaler device must only be wiped clean with a dry cloth after each use and is then replaced weekly.
"This is good news for the CF community," said Dr. Robert J. Beall, President and CEO, Cystic Fibrosis Foundation. "Managing daily CF treatments is a challenge for people with CF. TOBI Podhaler helps relieve that burden by shortening the time it takes to administer the medicine and making it easy for people with CF to take their treatment with them wherever they need to go."




