Medtronic has announced that the company has received U.S. Food and Drug Administration (FDA) approval for the CareLink SmartSync(TM) Device Manager.
With the introduction of SmartSync, physicians will now be able to use an Apple® iPad® to program and manage data from Medtronic's BlueSync-enabled implanted cardiac devices.
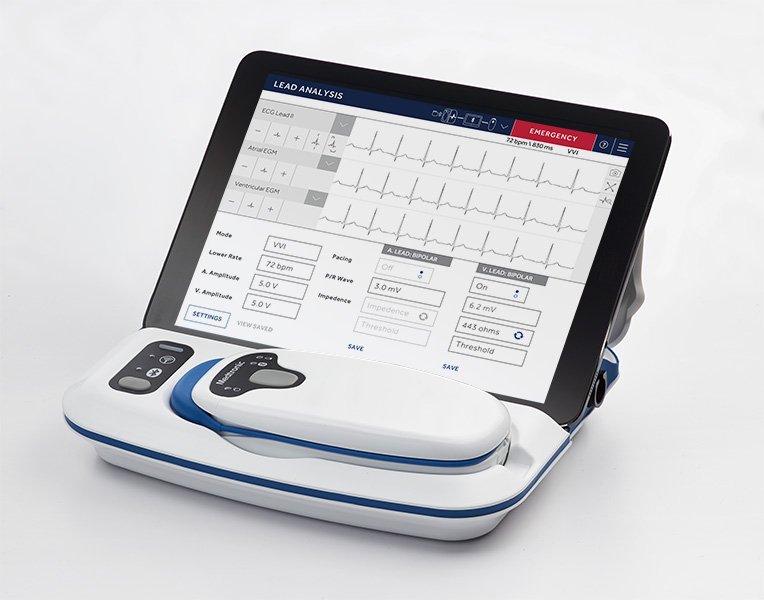
SmartSync is a next-generation programmer and pacing system analyzer that follows the popular CareLink(TM) 2090 programmer. It features a simplified user interface, enhanced security and Bluetooth® capabilities to communicate with compatible cardiac devices. As a wireless system that weighs just over two pounds, SmartSync is easily portable allowing physicians more freedom to engage with patients as they program and manage cardiac devices and device data. SmartSync includes the CareLink SmartSync mobile application, patient connector (telemetry head) and base station (pacing system analyzer).
Rob Kowal, CMO, Vice President of medical affairs and business development of the Cardiac Rhythm and Heart Failure division, which is part of the Cardiac and Vascular Group at Medtronic said, “Medtronic is committed to developing smart technology solutions that help physicians seamlessly provide high-quality care to cardiac patients. SmartSync is another important innovation reinforcing that commitment. Clinicians will benefit from the portability that SmartSync offers, and because it was built to replicate the familiar CareLink 2090 interface, the transition to this new technology is quite simple."
SmartSync is the latest smart technology to join Medtronic's cardiac care management platform, which not only boasts the CareLink(TM) network, the leading remote monitoring system for cardiac patient data, but also includes the MyCareLink Heart(TM)mobile app.
Launched in January 2019, the MyCareLink Heart mobile app allows patients to use smart technology with Bluetooth capabilities to communicate with compatible BlueSync-enabled cardiac devices. BlueSync technology-enabled pacemakers include the Azure(TM) pacemaker and Percepta(TM), Serena(TM) and Solara(TM) quadripolar cardiac resynchronization therapy pacemakers (CRT-Ps).
The SmartSync Device Manager received CE Mark approval in 2018 and is currently available in more than 20 countries worldwide. It is also available in select sites in the U.S. with a full U.S. launch expected in August 2019.
Klaus Witte, associate professor and consultant cardiologist, and lead clinician for cardiology at the University of Leeds and Leeds Teaching Hospitals NHS Trust in the United Kingdom said, "The wireless capabilities and data transfer options of the SmartSync Device Manager have increased my efficiency in the lab and allow me to interact with my patients and peers more directly without sacrificing performance. This type of portable device programing represents the future of cardiac data technology."
In addition to building SmartSync to provide physicians with unique data management benefits, Medtronic prioritized high-level security design capabilities. The security controls built into SmartSync and all BlueSync-enabled heart devices include multi-encryption, run time application monitoring for intrusion detection, and access restrictions designed to protect the device and data transmissions.
In collaboration with clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias.