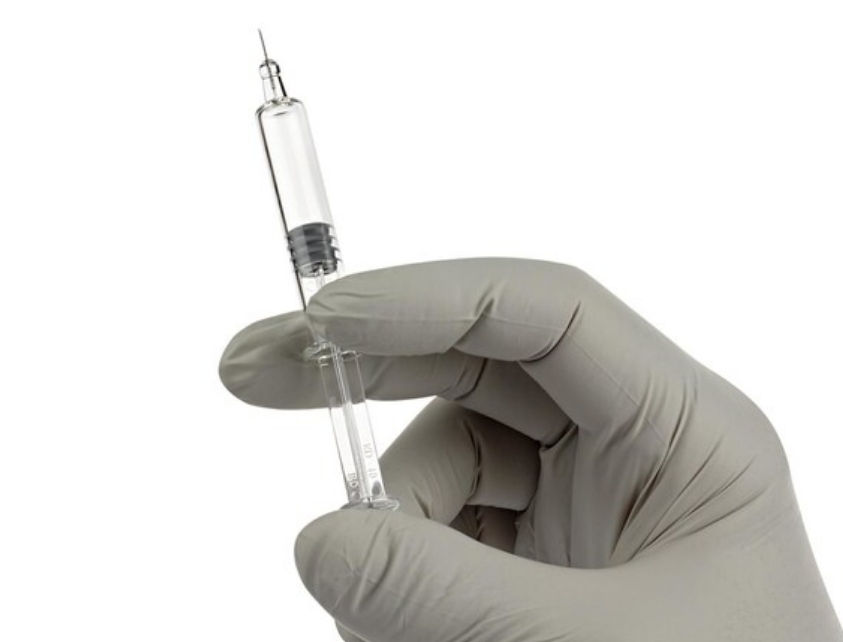
BD (Becton, Dickinson and Company), a leading global medical technology company, has announced the commercial release of the BD Neopak XtraFlow Glass Prefillable Syringe and the latest capacity expansion of the BD Neopak Glass Prefillable Syringe platform to serve the growing market for biologic therapies.
The BD Neopak Glass Prefillable Syringe platform is designed to address key development needs for biologic drugs and customers have received approval to utilise this platform for more than 24 indications, including Crohn's disease, atopic dermatitis, cardiovascular disease and various rare diseases.
BD has expanded usability of this platform with the commercial release of the BD Neopak XtraFlow Glass Prefillable Syringe. This solution features an 8-millimeter needle length and thinner wall cannula to optimize subcutaneous delivery of higher viscosity drug profiles by reducing the injection force and time required for a fixed solution viscosity. This improvement allows pharmaceutical developers to break design barriers, enhancing flow and usability beyond today's limits versus a standard half-inch needle.
In addition to the usability enhancements, BD's manufacturing site in Le Pont-de-Claix, France has successfully integrated a high-volume manufacturing line for the BD Neopak Glass Prefillable Syringe platform, increasing the production capacity of a single line by sevenfold.




