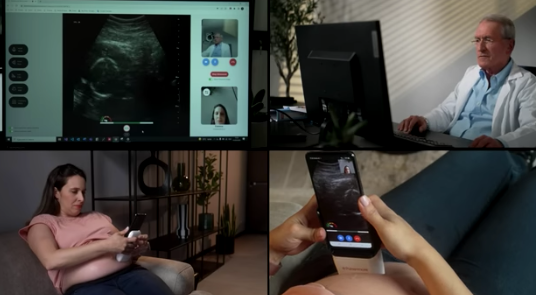
Israel-based startup Pulsenmore, the world leader in self-scan ultrasound technology for at-home use and remote clinical diagnosis, has announced its new clinical study collaboration with Michigan Medicine. The study will examine the ability to conduct a remote biophysical profile (BPP) test, by using the Pulsenmore home ultrasound with the guidance of a remote clinician, potentially reducing the clinical burden.
The Pulsenmore prenatal home ultrasound, empowers pregnant women to connect their personal smartphones to a dedicated device and application, allowing them to perform ultrasound imaging scans from the comfort of their homes. These scans are seamlessly transmitted to the hospital for evaluation, focusing on essential fetal vitality parameters. The results are then communicated back to the patients. Clinicians can engage with patients asynchronously or in real-time, significantly reducing the necessity for in-clinic visits.
Traditional antenatal testing, like the biophysical profile (BPP), typically conducted in clinical settings, involves assessing foetal parameters such as movement, tone, breathing, and fluid levels using ultrasound, and sometimes combined with a non-stress test.
The study with Michigan Medicine, the University of Michigan's academic medical center aims to assess whether patients can successfully complete a BPP using the Pulsenmore device with remote clinician guidance. The success of the study could potentially lead to reducing the burden of clinical care and improving patients' pregnancy experience due to factors like transportation, rural residence, or balancing childcare and work commitments.




