image credit- shutterstock
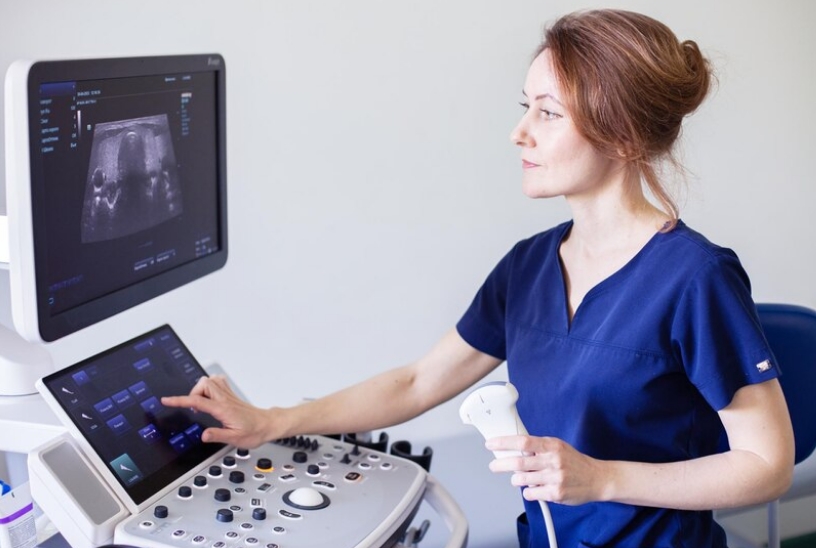
Australia-based startup See-Mode Technologies, a global innovator in artificial intelligence (AI) for ultrasound imaging, has announced the receipt of 510(k) clearance from the US Food and Drug Administration (FDA) for their thyroid ultrasound analysis and reporting software.
See-Mode's AI solution aims to reduce reporting time and variation in delivery of care for thyroid ultrasound. The software detects nodules in single or multinodular thyroid ultrasound images, and automatically classifies each nodule in line with the American College of Radiology's (ACR) TI-RADS rating systems.
A complete worksheet is automatically generated and preliminary impressions are sent to radiology reporting systems after clinician review and approval. Existing CPT codes relevant to the use of AI for analysis of thyroid ultrasound also provide greater reimbursement opportunities.
This is the first FDA-cleared product for both detection and diagnosis (CADe/x) of thyroid ultrasound. See-Mode differentiates its thyroid product with the level of automation that it offers, including automatic detection and characterization of thyroid nodules without manual user input, while enabling the clinicians to review and amend the AI outputs quickly before finalising the report. The product also streamlines reporting of follow-up thyroid studies, addressing a significant pain point and time-consuming task for radiologists.




