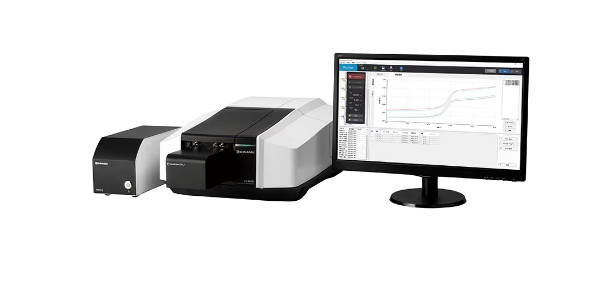
Japanese firm Shimadzu has released the Tm analysis system both in Japan and internationally. This analyses the thermal stability of nucleic acid pharmaceuticals in development using a UV-VIS spectrophotometer. This is the first system to automate the processes from measurement of the Tm value, a standard index of the thermal stability of nucleic acids, to data analysis.
The Tm value is indispensable for research and development in the nucleic acid pharmaceutical market, which is expected to have an annual growth rate of approximately 17% by 2030.
The Tm analysis system automatically performs everything from sample measurements to data analysis in one step. The system consists of a UV-VIS spectrophotometer in the UV-Vis series, the TMSPC-8i 8-series thermoelectrically cooled cell holder, and the LabSolutions UV-Vis Tm measurement analysis software. The newly developed micro cell limits evaporation of the sample, enabling trace quantity measurements, so precious nucleic acid samples are not wasted.
The newly designed software is compliant with standards related to data integrity (data integrity concomitant on measurement) as required in the pharmaceutical industry.



