Israel-based firm Medasense, a global leader in pain monitoring solutions, has announced a strategic partnership with Nihon Kohden for the exclusive distribution of its revolutionary pain monitoring device in Japan.
This partnership is intended to transform pain management practices across Japanese healthcare facilities, offering a significant advancement in patient care.
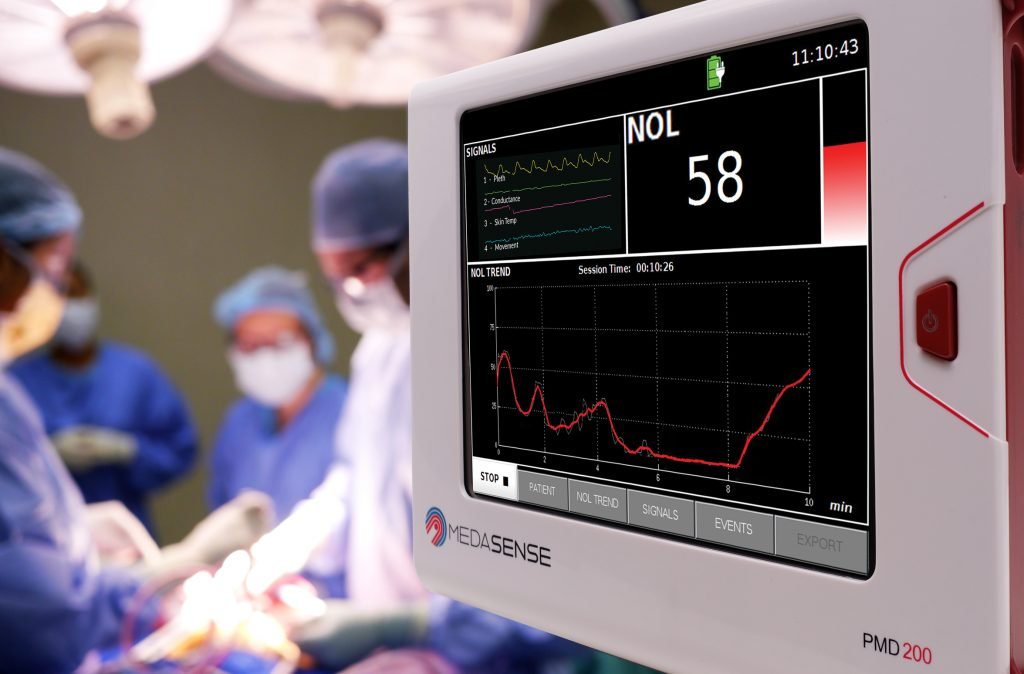
Nihon Kohden, renowned for its history of excellence in providing innovative high quality, reliable medical technology that improves the way healthcare is practiced, is partnering with Medasense to introduce its nociception monitor to the Japanese market. This cutting-edge device, with its state-of-the-art artificial intelligence (AI) powered NOL - Nociception Level Index, provides real-time, objective pain monitoring, enabling the personalisation and optimisation of pain treatment. It will be accessible to hospitals and clinics throughout Japan through Nihon Kohden's extensive distribution channels pending regulatory approval.
NOL monitoring provides an AI powered, clinically validated index to objectively quantify the physiological response to pain (nociception) supporting clinicians in delivering personalized anesthesia tailored to patient requirements. With over 40 peer reviewed publications, clinical studies have demonstrated that NOL-guided analgesia resulted in intraoperative opioid sparing, and improved post operative pain scores and patient recovery.
The company's flagship product, the PMD-200, equipped with the NOL-Nociception Level Index, leverages advanced artificial intelligence and a proprietary non-invasive sensor system. This unique platform provides objective monitoring and quantification of a patient's pain response, making it an essential tool in an operating room and critical care unit settings where patients cannot communicate their pain levels. The PMD-200 is the first and only monitor to be authorised by the US FDA for pain measurement for anaesthesiology. It has been used in over 100,000 surgeries worldwide, and is commercially available in the US, Europe, Canada, Latin America and Israel.




