Korean firm EOFlow’s innovative wearable insulin pump gets UAE approval
September 05, 2022 | Monday | News
Fast product approval after signing the exclusive distribution agreement with GulfDrug in GCC 6 countries in March
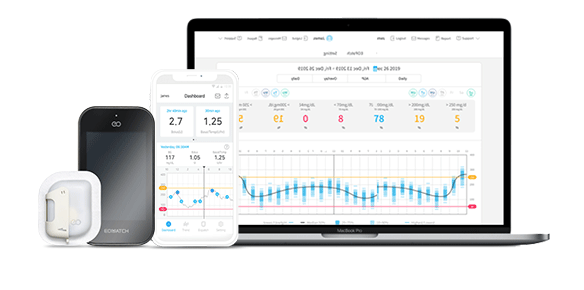
South Korea-based EOFlow, a provider of wearable drug delivery solutions, has received certification from the United Arab Emirate (UAE) Ministry of Health and Prevention (MOHAP) to commercialize its wearable insulin pump ‘EOPatch’ and its controller ‘ADM’ as well as its smartphone app ‘Narsha’.
With this certification, EOFlow will be able to sell its EOPatch insulin management system through online and offline distribution channels throughout the UAE. GulfDrug plans its full launch of EOPatch in 2H of 2022 supported with various sales promotions to build product awareness. GulfDrug also plans to set UAE as a sales base for the Middle Eastern market and begin its submission process of EOPatch to other Gulf Cooperation Council (GCC) members (Bahrain · Kuwait · Oman · Qatar · Saudi Arabia) in 2023.
According to the International Diabetes Federation (IDF) data from 2019, approximately one in six adults (1.2 million) are living with diabetes in the UAE, a prevalence of 16.3% which is significantly higher than Korea's 6.9% and the world average of 8.24%.
Diabetes and its complications account for 72.1% of main causes of death among Emiratis (citizens of the United Arab Emirates) under the age of 60, which suggests that most of the adult population is at risk of diabetes. The government's efforts to improve the health of the Emiratis with diabetes continue, creating a favorable market condition for EOFlow to expand its wearable pump business in the region.






