Theranica set to market acute migraine-relief wearable device
May 30, 2019 | Thursday | News
The FDA market authorization is based on the results of a prospective, randomized, double-blind, placebo-controlled, multi-center pivotal study, where 252 patients from 12 clinics used the non-invasive wearable to treat their migraine attacks
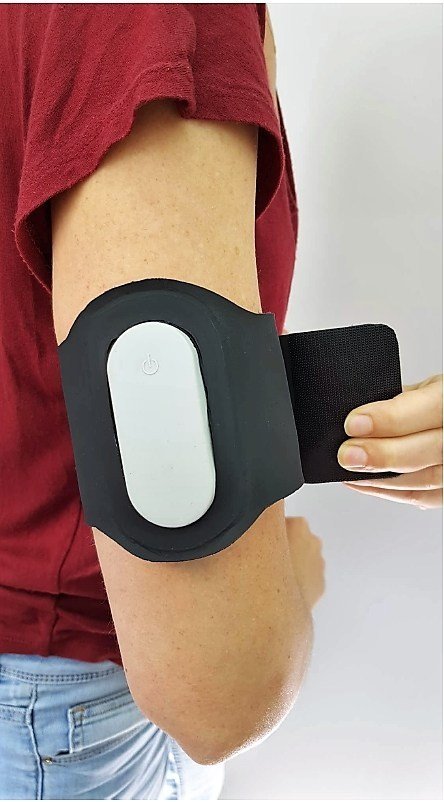
Theranica, a bio-medical technology company developing advanced electroceuticals for migraine and other pain disorders, announced today that the U.S. Food and Drug Administration (FDA) granted a De Novo request for its smartphone-controlled electroceutical, Nerivio Migra®, utilizing Remote Electrical Neuromodulation for the acute treatment of migraine.
"The clinical data of this innovative therapeutic device is of very high quality," commented Professor Messoud Ashina, Danish Headache Center, president-elect of the International Headache Society. "It indicates that the device can provide patients with significant relief of pain and other migraine symptoms without the side effects presented by drugs."
The FDA market authorization is based on the results of a prospective, randomized, double-blind, placebo-controlled, multi-center pivotal study, where 252 patients from 12 clinics used the non-invasive wearable to treat their migraine attacks.
Nerivio Migra®, a first-in-category product, is placed on the upper arm (not the head or neck) and uses smartphone-controlled electronic pulses to create a Conditioned Pain Modulation (CPM) response. Nerivio Migra® is indicated for acute treatment of migraine with or without aura in adult patients who do not have chronic migraine.
"While the company is preparing to launch the Nerivio Migra® in the United States market later this year at an affordable price, we remain committed to continuing our clinical development, expanding the use of remote electrical neuromodulation therapy for additional indications," said Alon Ironi, CEO and co-founder of Theranica. "We have identified at least 7 different painful conditions that may be relieved by this non-invasive, drug-free technology after appropriate clinical development."
"Physicians who treat people with migraine are both patient-centered and science-driven," said Prof. Stephen Silberstein, director of the Headache Center at the Jefferson University Hospital in Philadelphia, a member of the medical advisory board of Theranica. "Over the last 20 years my colleagues and I have used triptans and ergots for acute migraine treatment. There is a large unmet need for new treatments in this population when these medications are not effective, are contra-indicated, or have non-tolerable side effects. In addition, triptans and most current acute migraine medications, including over-the-counter drugs indicated for migraine, are associated with medication-overuse headache (MOH), which is associated with increased frequency of migraine attacks, and often results in chronic migraine. This new innovative FDA-authorized treatment is an important alternative to help our patients control this debilitating condition."






