

























Japanese pharmaceutical firm Daiichi Sankyo has established Daiichi Sankyo Research Institute in San Diego, US

The inauguration ceremony for the Fosun Pharma Middle East Office and Fosunhenlius Middle East United Company

Glenmark Specialty S.A. (GSSA), a wholly owned subsidiary of India-based Glenmark Pharmaceuticals, has entered

Australia’s biotech capital, Melbourne, has further strengthened its research and development capabiliti

Seoul's bio startup hub 'Seoul Bio Hub' and global pharmaceutical leader 'Celltrion' have joined forces to lau

MGI Tech, a company dedicated to developing core tools and technologies that drive innovation in life sciences

Japanese pharmaceutical company Kubota Pharmaceutical Holdings Co., Ltd. will introduce Thailand to

Singapore General Hospital (SGH) has announced the launch of a Centre of Excellence (CoE) for robotic-assisted
UK-based eXmoor Pharma, the integrated cell and gene therapy CDMO with embedded consultancy expertise, and Tha

Celltrion Pharm, a South Korean biopharmaceutical firm, has inked a strategic trilateral agreement with a mult

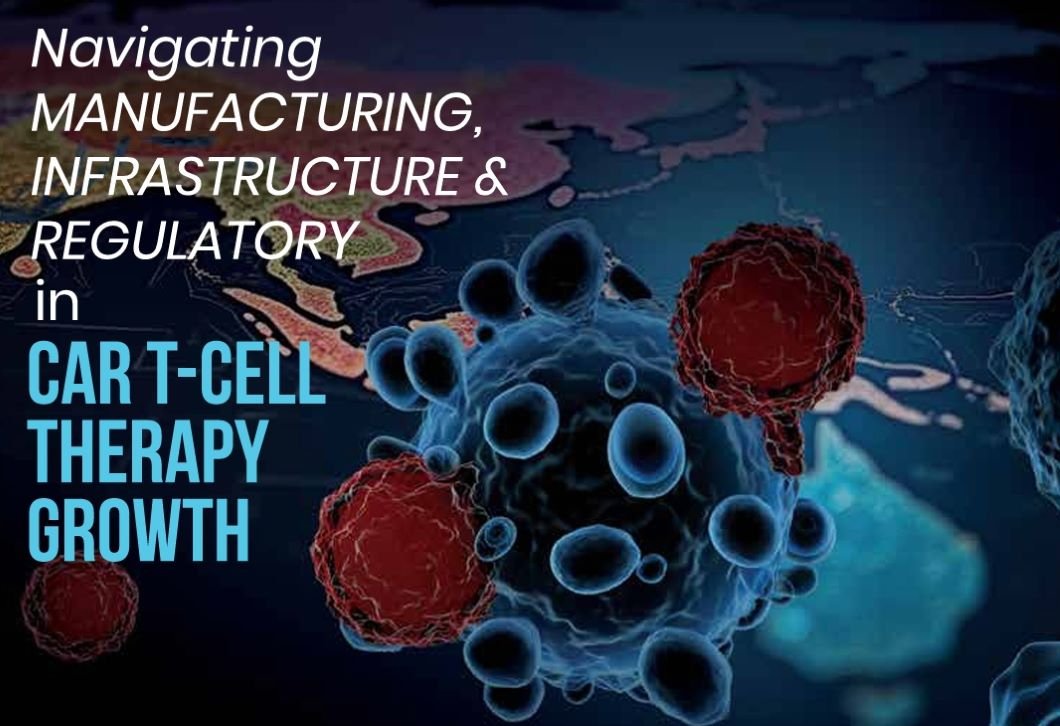
Navigating Manufacturing, Infrastructure & Regulatory Hurdles in CAR T-CELL THERAPY GROWTH
CAR T-cell therapy is a highly personalised treatment that depends on a unique and complex manufacturing and delivery process to reach patients quickly and reliably. From leukapheresis to final product delivery, the process is continuous and timesensitive, posing significant operational challenges. How is the industry addressing them, and what steps are needed to build a sustainable manufacturing ecosystem?
For Feedback, please email us at: communications@mmactiv.com
Dubai International Pharmaceutical & Technology Conference& Exhibition (DUPHAT)
Index Conferences & Exhibitions
info@duphat.ae https://duphat.ae/Dubai World Trade Center
Festival of Biologics 2025
Terrapinn
ewa.trojcewicz@terrapinn.com https://www.terrapinn.com/conference/festival-of-biologics/index.stmBasel
The MedTech Conference 2025
AdvaMed
conferenceinfo@advamed.org https://themedtechconference.com/San Diego



