
The fifth edition of the annual BioSpectrum Asia Pacific clinical research organization (CRO) Survey, which is conducted in association with CyberMedia Research (CMR), yet again identifies the latest trends, issues, perspectives and insights regarding the CRO industry in Asia Pacific (APAC).
While the 2013 survey revealed many hindrances facing the industry, including lack of optimum regulatory efficiency, training of personnel, limited development partnerships between CROs and pharma firms; it also reveals many promising findings, including high willingness to participate in clinical trials among countries like China, India, Australia, Korea, Taiwan, Malaysia, Philippines and Indonesia. Furthermore, the CRO sector in APAC is witnessing a major boom leveraging on the growth of logistics and supply chain management in the region.
The survey reveals that APAC CROs offer an array of services to their clients in the region. These services include, among others, patient and investigator recruitment, clinical trials monitoring, project management, bioanalytical laboratories, data intelligence and medical writing.
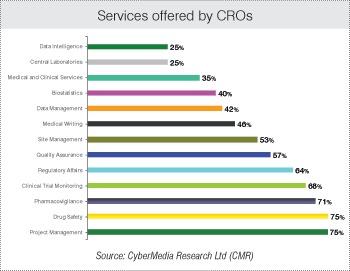
According to 75 percent respondents of the fifth BioSpectrum-CMR Asia Pacific Annual Survey of the Clinical and Contract Research Organizations 2013, their services are concentrated in project management.
As many as 71 percent of the respondents focus on pharmacovigilance; 68 percent indicate clinical trial monitoring, 64 percent in regulatory affairs; 57 percent focus on quality assurance, while 53 percent offer site management.




