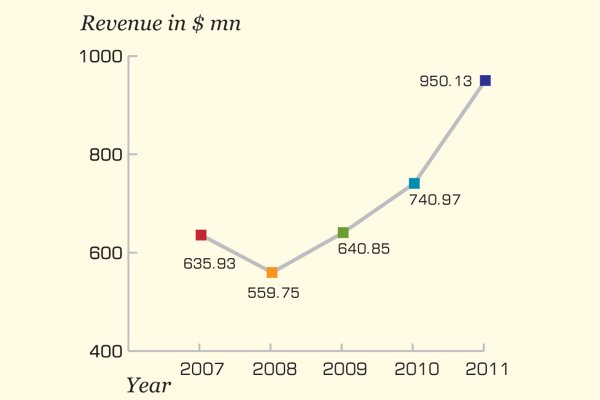
In 2011, the company's revenue witnessed a rise of 28 percent
 Dong-A Pharmaceutical adopts innovative and flexible business models to achieve substantial revenues at the end of each financial year. In 2011, the company's revenue witnessed a rise of 28 percent, from $741 million in 2010 to $950 million in 2011. The firm followed the business model of entering into strategic licensing agreements with worldwide players and establishing collaborations with many companies.
Dong-A Pharmaceutical adopts innovative and flexible business models to achieve substantial revenues at the end of each financial year. In 2011, the company's revenue witnessed a rise of 28 percent, from $741 million in 2010 to $950 million in 2011. The firm followed the business model of entering into strategic licensing agreements with worldwide players and establishing collaborations with many companies.
Dong-A Pharma signed an agreement with Meiji Seika Pharma, Japan, to license out Zydena (udenafil) tablets, a popular daily treatment for erectile dysfunction. Moving ahead with its licensing strategies with other global players, Dong-A signed an agreement with the UK-based Medipost to gain the exclusive local sales rights of Cartistem, an articular cartilage restoration stem cell therapeutics.
The company also signed a licensing deal with Japan-based Mitsubishi Tanabe Pharma to develop, manufacture, and market Talion eye drops (bepotastine besilate), an allergic conjunctivitis treatment in South Korea with a market size of approximately $20.6 million, growing at 17 percent per year.
Dong-A has also signed an agreement with Meiji Seika Pharma on comprehensive collaboration to construct a biosimilar production plant in Songdo, South Korea, to target the global markets with antibody-based drugs. Dong-A also plans additional investment in Songdo to export biopharmaceutical products, such as PEG-G-CSF for neutropenia, interferon beta and diabetes treatments, which are presently in Dong-A quote s pipeline.
Not forgetting the local market, the company signed an agreement with Astellas for co-promoting Irribow (ramosetro) in various health centers across South Korea. On the domestic front, Dong-A concluded the acquisition of Samchully, a South Korean supplier of ingredients for drugs. In a bid to strengthen its position in the global pharmaceutical industry, Dong-A Pharma plans to reinforce its global competences, backed by strategic alliances. Dong-A Pharma aims to become a global pharmaceutical by increasing its market share in the major global biopharmaceutical markets.
It signed a licensing agreement with Medipost, regarding local sales rights for Cartistem. Selected as a Consumer Complaints Management System (CCMS) certified company up on the evaluation conducted by CCMS program, under Fair Trade Commission and The Organization. The company signed an agreement with Mitsubishi Tanabe Pharma, under which Dong-A shall be granted exclusive rights to develop, manufacture, and market Talion eye drops (bepotastine besilate), an allergic conjunctivitis treatment in South Korea. It also Signed an MoU with Incheon Free Economic Zone to develop a biopharmaceutical industry complex in Songdo.




