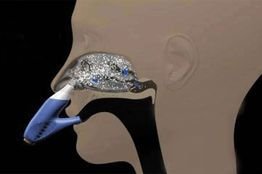
Intranasal delivery provides significant advantage over subcutaneous vaccine as a needle-free and painless vaccination
There is a fear that avian H5N1 virus could cause a pandemic of new influenza in humans without any warning. To prevent the contagious infectious diseases spreading in humans, it is important to develop a vaccine that induces cross-protective immunity against variant viruses.
In case of mucosally-acquired acute respiratory infection, such as influenza, mucosal immunity induced by natural infection plays important role in protection against the infection. An example is mucosal secretory IgA antibody that plays an important role in cross-protection.
At Japan's National Institute of Infectious Diseases, researchers have derived that an effective adjuvant is necessary to break tolerance for the development of inactivated intranasal vaccine. Cholera Toxin (CT) and E Coli heat labile Toxin (LT) have been used as mucosal adjuvant for intranasal influenza vaccine. However, CT and LT have some side effects, such as nasal discharge or Bell's palsy. Therefore, safer and effective mucosal adjuvant is required for human clinical application of intranasal influenza vaccine.
Mr Hideki Hasegawa, director, Department of Pathology, National Institute of Infectious Diseases, Japan, agrees that there is a threat of pandemic. "Subcutaneous injection of inactivated vaccines would be an effective strategy in an epidemic caused by a homologous virus, as it induces specific serum IgG, but would be less effective in an epidemic caused by a heterologous virus," he says. "On the other hand, live attenuated vaccines effectively protect against heterologous virus infection by inducing secretory IgA, IgG, and cytotoxic lymphocyte (CTL) responses. However, safety has been proven only in healthy people between the age of five years and 49 years. Intranasal administration of inactivated vaccines represents a potential solution to overcome these problems. Intranasal administration of inactivated vaccine plus adjuvant or live attenuated vaccines are promising candidates for inducing cross-protective immunity against variant influenza viruses."
Intranasal delivery of vaccine provides significant advantage over subcutaneous vaccine as a needle-free and painless vaccination. The nasal mucosa provides a convenient surface for vaccine deposition and for induction of systemic and local mucosal immunity. Researchers have shown that antibodies induced by intranasal vaccination are effective in preventing infection and also protects the pulmonary tract in a therapeutic manner after pathogen exposure. In response to the demand of innovative drug delivery platform, intranasal vaccines scores high.
Making advances in the development of vaccine delivery platforms, AstraZeneca launched intranasal vaccine FluMist, prescribed for age group of two to 50 years, for seasonal influenza in 2003. Later, introducing the breakthrough drug delivery technology in India, Serum Institute of India (SII) developed India's first intranasal vaccine Nasovac against H1N1 virus in 2010, at a much cheaper price than other available H1N1 vaccines.
Australia's Walter and Eliza Hall Institute and Royal Melbourne Hospital are conducting human trial of nasal spray vaccine for type 1 diabetes. Researchers have demonstrated that when administered through the nasal passages, the insulin vaccine stimulates the immune system present in the mucosal linings and works to desensitize the whole immune system. The nasal vaccine approach, if shown to be successful in human type 1 diabetes, could also be tested with different vaccines for the prevention of other autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis, say researchers at Walter and Eliza Hall Institute.




