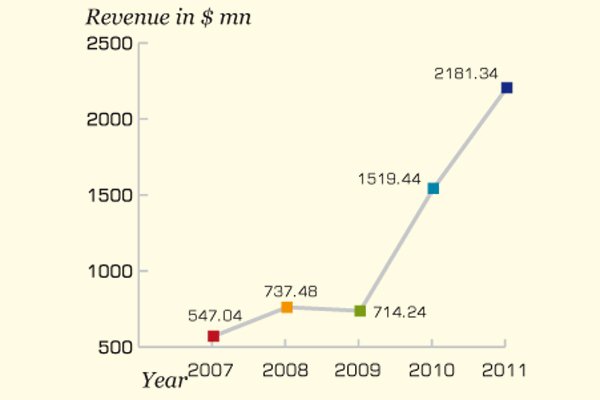
The company witnessed the growth of 43.56 percent in 2011
 North China Pharmaceutical Group (NCPC) is a nationwide provider of bulk pharmaceuticals in China, including antibiotics, pharmaceutical intermediates, nutraceuticals, veterinary products, semi-synthetic antibiotics, biotechnology products, and vitamins. It also offers finished preparations, which comprise capsules, granules and dry syrups, injections, lyophilized powders, oral solutions, powders for injection, and tablets.
North China Pharmaceutical Group (NCPC) is a nationwide provider of bulk pharmaceuticals in China, including antibiotics, pharmaceutical intermediates, nutraceuticals, veterinary products, semi-synthetic antibiotics, biotechnology products, and vitamins. It also offers finished preparations, which comprise capsules, granules and dry syrups, injections, lyophilized powders, oral solutions, powders for injection, and tablets.
The annual sales volume for antibiotic bulks is up to 8,000 tons, for vitamins it is 23,000 tons, for antibiotic intermediates 5,000 tons, and for formulations it amounts to 4.1 billion vials or tablets or capsules; and the main products now occupy a considerable share of the domestic market. The output and sales volume of penicillin, amoxicillin, streptomycin sulfate, cefradine, 7-ADCA, vitamin C, and vitamin B12 are ranked high.
The growth in the volume of products sold by NCPC supported the growth in the revenue as well. The company witnessed the growth of 43.56 percent in 2011 as the sales revenue for 2011 reached $2.18 billion from $1.52 billion in 2010.
NCPC has developed strong production base for antibiotics with advanced technology, and is recognized as a leading antibiotics producer in the country, securing its position among top 500 enterprises in China. Expanding its business, NCPC is closely cooperating with foreign companies and institutions, and the company has established 16 joint and cooperative ventures.
At present, NCPC is the second largest penicillin enterprise in the world, and is the largest manufacture of amoxicillin, streptomycin, cefradine, 6-APA and 7-ADCA in China. Based on the company's new strategy, NCPC is now developing biopharmaceuticals.
*Q4 results are extrapolated
Utilizing its technological advantages accumulated in the field of traditional fermentation, NCPC has initiated modern biotech research and has set up a biotech drugs development system. NCPC has launched G-CSF, GM-CSF, EPO and genetically engineered hepatitis B vaccines into the market.
Capturing the international market, NCPC's lincomycin hydrochloride, amphotericin B, and oxytetracycline have been approved by FDA. Escalating the quality of products to international standards, NCPC has built its manufacturing capacity strictly in compliance with international standards. NCPC has established its own internal quality control standards, which are much stricter than mandatory standards.
Gradually moving ahead, NCPC is transforming from generics to innovative drug development.




