The various types of staff needed to run a CRO

The fifth edition of the annual BioSpectrum Asia Pacific clinical research organization (CRO) Survey, which is conducted in association with CyberMedia Research (CMR), yet again identifies the latest trends, issues, perspectives and insights regarding the CRO industry in Asia Pacific (APAC).
While the 2013 survey revealed many hindrances facing the industry, including lack of optimum regulatory efficiency, training of personnel, limited development partnerships between CROs and pharma firms; it also reveals many promising findings, including high willingness to participate in clinical trials among countries like China, India, Australia, Korea, Taiwan, Malaysia, Philippines and Indonesia. Furthermore, the CRO sector in APAC is witnessing a major boom leveraging on the growth of logistics and supply chain management in the region.
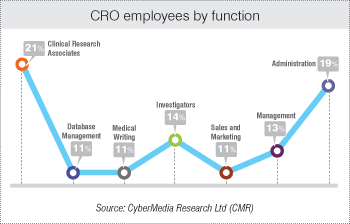
According to the survey results of the fifth BioSpectrum Asia - CMR Asia Pacific Annual Survey of the Clinical and Contract Research Organizations 2013, 21 percent of the employees in a CRO are clinical research associates (CRAs). Database Management is performed by 11 percent of the employees and 25 percent of the staff are focus on medical writing and clinical investigation.
Of the total workforce, 43 percent comprises of the sales, management and administration. These results are in line with the trends captured by earlier BioSpectrum Asia-CMR surveys.




