BioSpectrum Asia Top 20 Survey Rank 4 - China National Accord Medicines, China

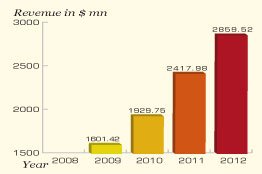
China National Accord Medicines Corporation utilized 2012 as a year to speed up the transformation and upgrade scientific development by implementing new medical reforms fostered by Chinese pharmaceuticals industry. China National Accord Medicines Corporation (also known as Sinopharm Accord) has four integrated businesses divisions, including medicine distribution, pharmaceutical development and pharmaceutical logistic. Sinopharm Accord marked the year with a steady year-on-year growth of a little over 18 percent and its pharmaceutical division scaled a growth of 2.33 percent.
In 2012, the pharmaceutical division of Sinopharm Accord established a development strategy named "123". It included setting up one industry platform of unified brand, creating a R&D center and a marketing center, and constructing three bases, including a raw material production base in Suzhou, a preparation production base in Guanglan, and an integrated preparation base in Pingshan.
Shenzhen Zhijun Pharmaceutical, the core enterprise of pharmaceutical division, has established scientific research cooperation with Sichuan Industrial Institute of Antibiotics and the division has extended product lines and developed corollary equipment for the production of crude drug.
Its distribution business, focused on smart supply chain creation, has brought changes in business strategies and gained significant achievement in developing its smart supply chain, improving distribution network layout, thereby surpassing its own benchmark and implementing service transformation. The company continued to spread the distribution network, enhanced its logistics network and strengthened core competition advantage by strengthening its logistic services all across China.
Sinopharm Accord established R&D strategy for generic drugs and defined the pattern and orientation for independent innovation, thus developing its R&D base. The company aims to further strengthen the integration advantage of R&D industrial chain of raw material-preparation and optimize resource allocation. The company's R&D center has actively implemented a strategy with 14 drugs in pipeline in 2012, nine drugs have been approved and total seven projects have been initiated, mainly concentrating on special drugs and differentiation development of antibiotics varieties. Pharmaceutical division has obtained 10 production approval documents for new products in 2012, eight projects for production declaration and seven for clinical declaration.




