Cell and gene therapy (CGT) is at the frontier of medical innovation, promising transformative solutions for some of the most challenging diseases. According to Novotech’s whitepaper, "Cell and Gene Therapies – Global Clinical Trial Landscape (2024)," the sector is experiencing unparalleled growth, fueled by breakthroughs in research, supportive regulatory environments, and global collaboration. Below, we delve into the key insights shaping the CGT landscape and what they mean for the future of healthcare.
From 2019 to 2023, the global clinical trial landscape for cell and gene therapies grew by an impressive 32.5%, with over 1,500 drug candidates under development spanning phases 0 to III. The Asia-Pacific region now leads in trial activity, accounting for 44% of all trials. China, in particular, drives this growth due to favorable regulations, cost efficiencies, and robust infrastructure, followed closely by the United States, which holds 85% of North American trials.
Key Drivers:
The scope of CGT is expanding to include novel technologies and targets. Beyond hematologic malignancies, gene therapies are being explored for rare genetic disorders like Duchenne Muscular Dystrophy and Sickle Cell Disease. Emerging approaches such as CAR-NK (natural killer) therapies offer reduced toxicity and hold potential for overcoming the immunosuppressive tumor microenvironment, particularly in solid tumors.
Technological Enablers:
The Competitive Geography of CGT
While China leads in trial numbers, the United States remains a dominant force in CGT innovation, particularly in market adoption and cutting-edge R&D. Europe also plays a crucial role, with key contributions from countries like the UK and Germany, which are pivotal in regulatory harmonization and collaborative clinical studies. However, the Asia-Pacific region outshines others in patient recruitment efficiency, averaging 16.07 months compared to 21.15 months in the U.S. and 18.5 months in Europe. This advantage underscores the region’s capability in executing trials at a faster pace.
Faster recruitment timelines in Asia-Pacific are attributed to several factors:
| Region | Clinical Trials (%) | Recruitment Timelines (Months) |
|---|---|---|
| China | 56 | 15.8 |
| United States | 85 | 21.15 |
| Europe (UK, Germany) | 20 | 18.5 |
| Asia-Pacific (Overall) | 44 | 16.07 |
The following chart visually compares recruitment timelines across regions:
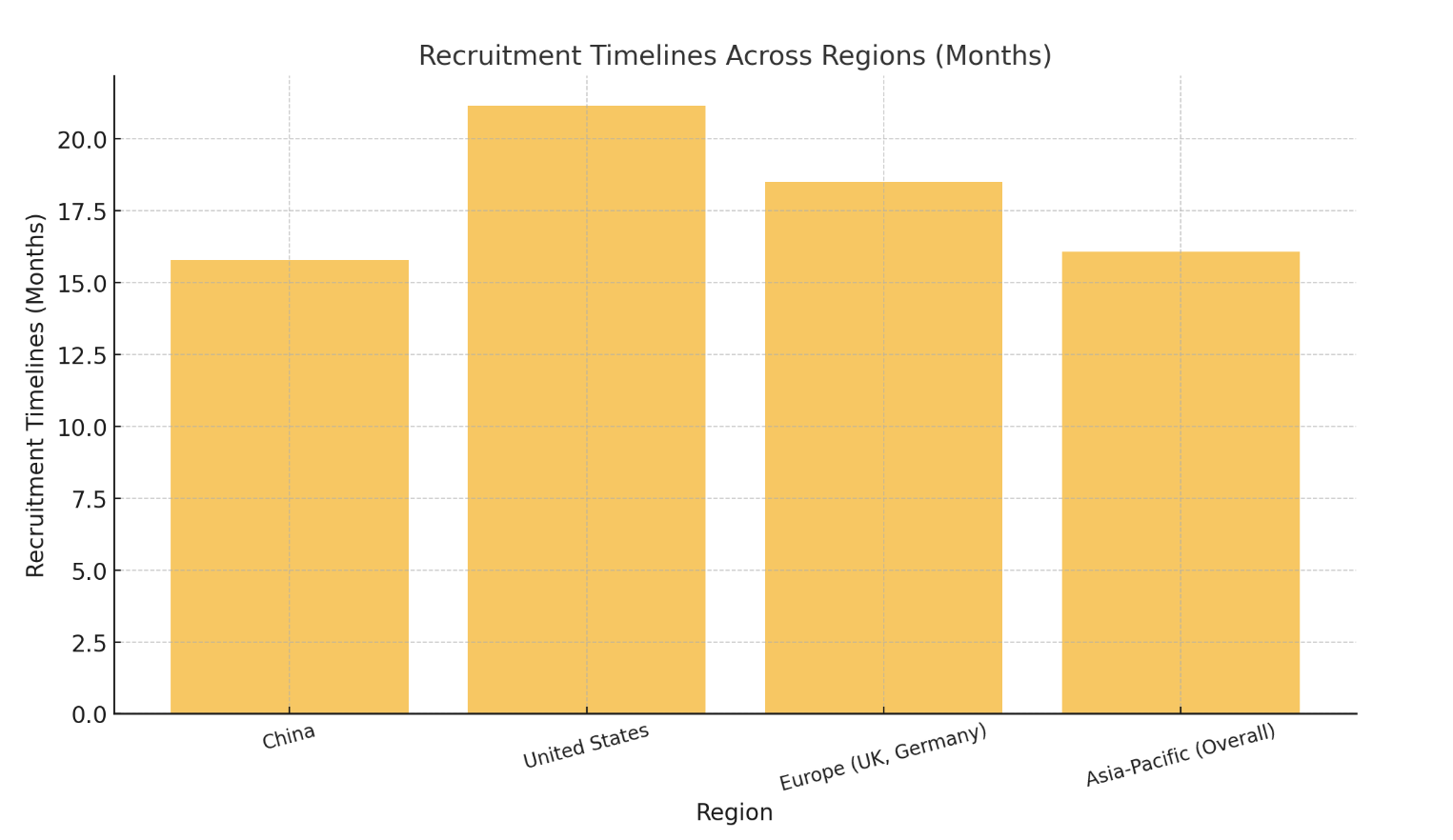
Asia-Pacific’s efficiency and cost-effectiveness make it an attractive hub for CGT development, enabling sponsors to accelerate timelines and optimize resources while expanding access to innovative therapies. This regional advantage is expected to drive continued growth and global partnerships in the years ahead.
While the potential of CGT is vast, several challenges persist, including high costs, complex regulatory landscapes, and scalability issues. Transitioning from autologous to allogeneic therapies—where treatments are "off-the-shelf" rather than patient-specific—could address some of these hurdles. However, ensuring batch consistency and regulatory compliance remains critical.
Strategic Pathways Forward:
By 2025, the FDA anticipates 10-20 CGT approvals annually, with over one million patients expected to benefit globally by 2034. As CGT transitions from experimental to mainstream medicine, stakeholders must balance innovation with operational pragmatism. Scaling up production, leveraging AI, and fostering global collaboration will be critical in unlocking the full potential of these therapies.
The CGT landscape exemplifies a dynamic confluence of science, technology, and strategy. As we navigate this era of medical breakthroughs, the collective efforts of researchers, developers, and regulators promise a future where previously untreatable diseases can be cured, offering hope to millions worldwide.




